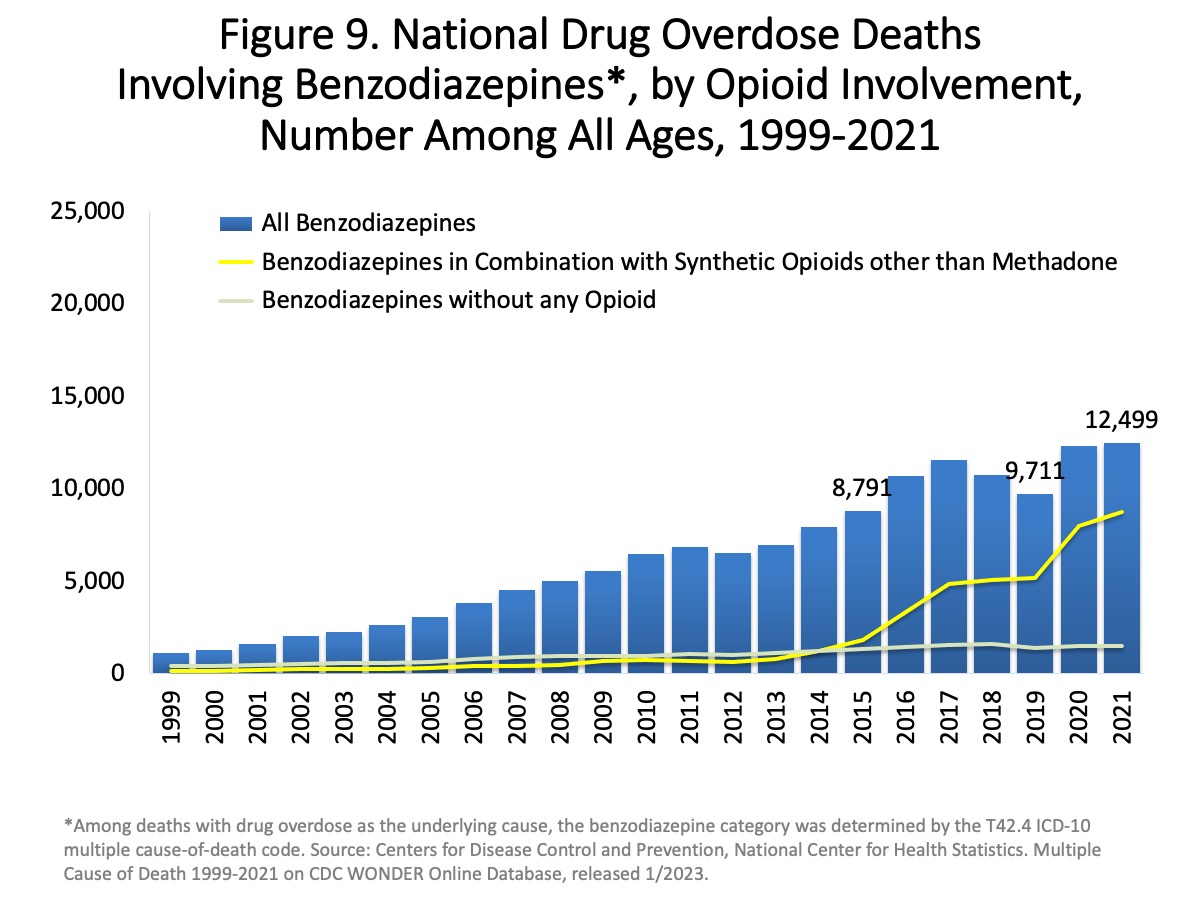
|
Metopon
Metopon (5-methylhydromorphone) is an opioid analogue that is a methylated derivative of hydromorphone which was invented in 1929 as an analgesic. Metopon is sometimes used in medicine, although longer acting than hydromorphone, Metopon is less potent and its oral bioavailability is fairly low. Generally, Metopon has few advantages to distinguish it from other, more commonly used opioid analgesics, although it does have a slightly lower tendency to produce nausea and respiratory depression compared to morphine. In Canada, as of 1948, the hydrochloride of metopon (free base conversion ratio 0.891, molecular weight 335.8) was available only for oral administration for malignant pain and for maintenance of those habituated to morphine; the only dosage form available was singly scored 8 mg tablets. It was manufactured by Parke, Davis, & Co., and was only for sale to doctors and hospitals. Parke, Davis & Co. did not sell metopon to pharmacies. It is unknown whether metopon tabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metopon Synthesis
Metopon (5-methylhydromorphone) is an opioid analogue that is a methylated derivative of hydromorphone which was invented in 1929 as an analgesic. Metopon is sometimes used in medicine, although longer acting than hydromorphone, Metopon is less potent and its oral bioavailability is fairly low. Generally, Metopon has few advantages to distinguish it from other, more commonly used opioid analgesics, although it does have a slightly lower tendency to produce nausea and respiratory depression compared to morphine. In Canada, as of 1948, the hydrochloride of metopon (free base conversion ratio 0.891, molecular weight 335.8) was available only for oral administration for malignant pain and for maintenance of those habituated to morphine; the only dosage form available was singly scored 8 mg tablets. It was manufactured by Parke, Davis, & Co., and was only for sale to doctors and hospitals. Parke, Davis & Co. did not sell metopon to pharmacies. It is unknown whether metopon tabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, and suppressing cough. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal. Opioids can cause death and have been used for executions in the United States. Side effects of opioids may include itchiness, sedation, nausea, respiratory depression, constipation, and euphoria. Long-term use can cause tolerance, meaning that increased doses are required to achieve the same effect, and physical dependence, meaning that abruptly discontinuing the drug leads to unpleasant withdrawal symptoms. The euphoria attracts recreational use, and frequent, escalating recreational use of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydromorphone
Hydromorphone, also known as dihydromorphinone, and sold under the brand name Dilaudid among others, is an opioid used to treat moderate to severe pain. Typically, long-term use is only recommended for pain due to cancer. It may be used by mouth or by injection into a vein, muscle, or under the skin. Effects generally begin within half an hour and last for up to five hours. Common side effects include dizziness, sleepiness, nausea, itchiness, and constipation. Serious side effects may include abuse, low blood pressure, seizures, respiratory depression, and serotonin syndrome. Rapidly decreasing the dose may result in opioid withdrawal. Generally, use during pregnancy or breastfeeding is not recommended. Hydromorphone is believed to work by activating opioid receptors, mainly in the brain and spinal cord. Hydromorphone 2 mg IV is equivalent to approximately 10 mg morphine IV. Hydromorphone was patented in 1923. It is on the World Health Organization's List of Ess ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CAS Number
A CAS Registry Number (also referred to as CAS RN or informally CAS Number) is a unique identification number assigned by the Chemical Abstracts Service (CAS), US to every chemical substance described in the open scientific literature. It includes all substances described from 1957 through the present, plus some substances from as far back as the early 1800s. It is a chemical database that includes organic and inorganic compounds, minerals, isotopes, alloys, mixtures, and nonstructurable materials (UVCBs, substances of unknown or variable composition, complex reaction products, or biological origin). CAS RNs are generally serial numbers (with a check digit), so they do not contain any information about the structures themselves the way SMILES and InChI strings do. The registry maintained by CAS is an authoritative collection of disclosed chemical substance information. It identifies more than 182 million unique organic and inorganic substances and 68 million protein and DNA seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
14-Methoxymetopon
14-Methoxymetopon is an experimental opioid drug developed by a team led by Professor Helmut Schmidhammer at the University of Insbruck in the mid 1990s. It is a derivative of metopon in which a methoxy group has been inserted at the 14-position. It is a highly potent analgesic drug that is around 500 times stronger than morphine when administered systemically; however, when given spinally or supraspinally, it exhibits analgesic activity up to a million fold greater than morphine. It binds strongly to the μ-opioid receptor and activates it to a greater extent than most similar opioid drugs. This produces an unusual pharmacological profile, and although 14-methoxymetopon acts as a potent μ-opioid full agonist in regard to some effects such as analgesia, a ceiling effect is seen on other effects such as constipation and respiratory depression which is believed to involve interaction with the κ-opioid receptor The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
14-Ethoxymetopon
14-Ethoxymetopon is an opioid analog that is a derivative of metopon which has been substituted with an ethoxy group at the 14-position. It is a highly potent analgesic drug several hundred times more potent than morphine Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies (''Papaver somniferum''). It is mainly used as a analgesic, pain medication, and is also commonly used recreational drug, recreationally, or to make .... References Semisynthetic opioids 4,5-Epoxymorphinans Phenols Ketones Ethers Mu-opioid receptor agonists {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
14-Phenylpropoxymetopon
14-Phenylpropoxymetopon (PPOM) is an opiate analogue that is a derivative of metopon which has been substituted with a γ-phenylpropoxy group at the 14-position. PPOM is a highly potent analgesic drug several thousand times stronger than morphine, with an even higher ''in vivo'' potency than etorphine. The 14-phenylpropoxy substitution appears to confer potent μ-opioid agonist activity, even when combined with substitutions such as N-cyclopropyl or N-allyl, which normally result in μ-opioid antagonist compounds. It has never been used in humans, but would be expected to produce effects similar to those of other potent opioid agonists, including strong analgesia, sedation, euphoria, constipation, itching and respiratory depression which could be harmful or fatal. Tolerance and dependence would be expected to develop rapidly based on the potency of the drug, as it is of a similar strength to the most potent of fentanyl analogues and so would most likely cause pronounced tachyph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Phenethyl-14-ethoxymetopon
''N''-Phenethyl-14-ethoxymetopon is a drug that is a derivative of metopon. It is a potent analgesic, around 60 times stronger than morphine and produces significantly less constipation. ''N''-Phenethyl-14-ethoxymetopon acts as an agonist at both μ- and δ-opioid receptors, with a Ki of 0.16nM at μ and 3.14nM at δ. See also * 14-Cinnamoyloxycodeinone * 14-Phenylpropoxymetopon * 7-PET * MR-2096 * N-Phenethylnormorphine * Phenomorphan * RAM-378 * Ro4-1539 Ro4-1539 (furethylnorlevorphanol) is an opioid analgesic drug from the morphinan series that was discovered by the pharmaceutical company Hoffmann–La Roche in the 1950s. It acts as a potent μ-opioid agonist, and was found to be around 30-60 t ... References Delta-opioid receptor agonists 4,5-Epoxymorphinans Phenols Ketones Ethers Mu-opioid receptor agonists Semisynthetic opioids {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered ret ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |