|
Methylecgonidine
Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester; AEME) is a chemical intermediate derived from ecgonine or cocaine. Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form methylecgonidine as a metabolite. Methylecgonidine has a relatively short half-life of 18–21 minutes, after which it is metabolised to ecgonidine, meaning that the relative concentrations of the two compounds can be used to estimate how recently crack cocaine has been smoked. Methylecgonidine has been shown to be specifically more harmful to the body than other byproducts of cocaine; for example to the heart, lungs & liver. The toxicity is due to a partial agonist effect at M1 and M3 muscarinic receptors, leading to DNA fragmentation and neuronal death by apoptosis. AEME is also used in scientific research for the manufacture of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylecgonidine Synthesis 1
Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester; AEME) is a chemical intermediate derived from ecgonine or cocaine. Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form methylecgonidine as a metabolite. Methylecgonidine has a relatively short half-life of 18–21 minutes, after which it is metabolised to ecgonidine, meaning that the relative concentrations of the two compounds can be used to estimate how recently crack cocaine has been smoked. Methylecgonidine has been shown to be specifically more harmful to the body than other byproducts of cocaine; for example to the heart, lungs & liver. The toxicity is due to a partial agonist effect at M1 and M3 muscarinic receptors, leading to DNA fragmentation and neuronal death by apoptosis. AEME is also used in scientific research for the manufacture of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylecgonidine Synthesis 3
Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester; AEME) is a chemical intermediate derived from ecgonine or cocaine. Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form methylecgonidine as a metabolite. Methylecgonidine has a relatively short half-life of 18–21 minutes, after which it is metabolised to ecgonidine, meaning that the relative concentrations of the two compounds can be used to estimate how recently crack cocaine has been smoked. Methylecgonidine has been shown to be specifically more harmful to the body than other byproducts of cocaine; for example to the heart, lungs & liver. The toxicity is due to a partial agonist effect at M1 and M3 muscarinic receptors, leading to DNA fragmentation and neuronal death by apoptosis. AEME is also used in scientific research for the manufacture of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylecgonidine Synthesis 2
Methylecgonidine (anhydromethylecgonine; anhydroecgonine methyl ester; AEME) is a chemical intermediate derived from ecgonine or cocaine. Methylecgonidine is a pyrolysis product formed when crack cocaine is smoked, making this substance a useful biomarker to specifically test for use of crack cocaine, as opposed to powder cocaine which does not form methylecgonidine as a metabolite. Methylecgonidine has a relatively short half-life of 18–21 minutes, after which it is metabolised to ecgonidine, meaning that the relative concentrations of the two compounds can be used to estimate how recently crack cocaine has been smoked. Methylecgonidine has been shown to be specifically more harmful to the body than other byproducts of cocaine; for example to the heart, lungs & liver. The toxicity is due to a partial agonist effect at M1 and M3 muscarinic receptors, leading to DNA fragmentation and neuronal death by apoptosis. AEME is also used in scientific research for the manufacture of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ecgonine
Ecgonine (tropane derivative) is a tropane alkaloid found naturally in coca leaves. It has a close structural relation to cocaine: it is both a metabolite and a precursor, and as such, it is a controlled substance in many jurisdictions, as are some substances which can be used as precursors to ecgonine itself. Structurally, ecgonine is a cycloheptane derivative with a nitrogen bridge. It is obtained by hydrolysis of cocaine with acids or alkalis, and crystallizes with one molecule of water, the crystals melting at 198–199 °C. It is levorotary, and on warming with alkalis gives iso-ecgonine, which is dextrorotary. It is a tertiary base, and has the properties of an acid and an alcohol. It is the carboxylic acid corresponding to tropine, for it yields the same products on oxidation, and by treatment with phosphorus pentachloride is converted into anhydroecgonine, C9H13NO2, which, when heated to 280 °C with hydrochloric acid, eliminates carbon dioxide and yields tro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ecgonidine
Ecgonidine (anhydroecgonine) is an alkaloid related to ecgonine and cocaine. It has a structure with a cycloheptene ring, with a nitrogen bridge, and a carboxylic acid side chain. Methylecgonidine is produced by pyrolysis in the process of smoking crack cocaine, and then subsequently metabolised to ecgonidine, and so these two compounds can be tested for as a specific biomarker for crack cocaine use. Ecgonidine is formed as a metabolite of methylecgonidine, and so the relative concentrations of the two compounds can be used to gauge how recently crack cocaine was smoked. If levels of methylecgonidine are higher, then the drug was smoked recently; however after a longer time period mainly ecgonidine will be present. Ecgonidine has a half-life in the body of around 94–137 minutes, and so can be used to detect use of crack cocaine up to 8–10 hours after the drug is consumed. See also * Coca alkaloids * Ecgonine * Tropacocaine Tropacocaine (tropacaine, benzoylpseudotropine, ps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloropane
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 0.79 and 18 nM, respectively. In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine. Methylecgonidine is the direct precursor to this compound. Trans -CO2Me group The thermodynamic isomer with a trans -CO2Me group is still active. This isomer was used by Neurosearch to make three different phenyltropanes which were tested in clinical trials. * Tesofensine * Brasofensine * NS-2359 (GSK-372,475) See also * 3,4-DCMP * O-2390 * List of cocaine analogues This is a list of cocaine analogues. A cocaine analogue is an (usually) artificial construct of a novel chemical compound from (often the starting point of natural) cocaine's molecular structure, with the result product sufficiently similar to co . ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Cyanoborohydride
Sodium cyanoborohydride is the chemical compound with the formula Sodium, NaBoron, BHydrogen, H3cyanide, CN. It is a colourless salt, but commercial samples can appear tan. It is widely used in organic synthesis for the reduction of imines. The salt tolerates aqueous conditions. Use Owing to the presence of the electron-withdrawing cyanide substituent, [B(CN)H3]− is less reducing than is sodium borohydride, [BH4]−. As a mild reducing agent, it is used to convert imines to amines. It is especially favored for reductive aminations, wherein aldehydes or ketones are treated with an amine in the presence of this reagent: : R2CO + R'NH2 + NaBH3CN + CH3OH → R2CH-NHR' + "NaCH3OBH2CN" The reagent is typically used in excess. Selectivity is achieved at mildly basic solutions (pH 7–10). The reagent is ideal for reductive aminations ("Borch Reaction"). In conjunction with tosylhydrazine, sodium cyanoborohydride is used in the reductive deoxygenation of ketones. Structure and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TBAF
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetrahydrofuran. TBAF is used as a source of fluoride ion in organic solvents. Preparation and properties TBAF can be prepared by passing hydrofluoric acid through an ion-exchange resin, followed by tetrabutylammonium bromide. Upon evaporation of the water, TBAF can be collected as an oil in quantitative yield. Preparing anhydrous samples is of interest as the basicity of fluoride increases by more than 20 p''K'' units on passing from aqueous to aprotic solvent. However, heating samples of the hydrated material to 77 °C under vacuum causes decomposition to the hydrogen difluoride salt. Similarly, samples dried at 40 °C under high vacuum still contain 10-30 mol% of water and some 10% of difluoride. Instead, anhydrous TBAF ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilkinson Catalyst
Wilkinson's catalyst is the common name for chloridotris(triphenylphosphine)rhodium(I), a coordination complex of rhodium with the formula hCl(PPh3)3(Ph = phenyl). It is a red-brown colored solid that is soluble in hydrocarbon solvents such as benzene, and more so in tetrahydrofuran or chlorinated solvents such as dichloromethane. The compound is widely used as a catalyst for hydrogenation of alkenes. It is named after chemist and Nobel laureate Sir Geoffrey Wilkinson, who first popularized its use. Historically, Wilkinson's catalyst has been a paradigm in catalytic studies leading to several advances in the field such as the implementation of some of the first heteronuclear magnetic resonance studies for its structural elucidation in solution (31P), parahydrogen-induced polarization spectroscopy to determine the nature of transient reactive species, or one of the first detailed kinetic investigation by Halpern to elucidate the mechanism. Furthermore, the catalytic and organome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
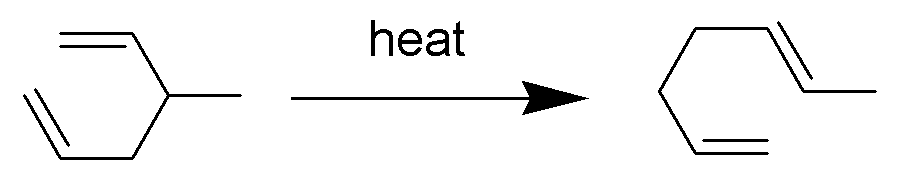
Cope Rearrangement
The Cope rearrangement is an extensively studied organic reaction involving the ,3sigmatropic rearrangement of 1,5-dienes. It was developed by Arthur C. Cope and Elizabeth Hardy. For example, 3-methyl-hexa-1,5-diene heated to 300 °C yields hepta-1,5-diene. The Cope rearrangement causes the fluxional states of the molecules in the bullvalene family. Mechanism The Cope rearrangement is the prototypical example of a concerted sigmatropic rearrangement. It is classified as a ,3sigmatropic rearrangement with the Woodward–Hoffmann symbol π2s+σ2s+π2s.html" ;"title="sub>π2s+σ2s+π2s">sub>π2s+σ2s+π2sand is therefore thermally allowed. It is sometimes useful to think of it as going through a transition state energetically and structurally equivalent to a diradical, although the diradical is not usually a true intermediate (potential energy minimum). The chair transition state illustrated here is preferred in open-chain systems (as shown by the Doering-Roth experiments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings. Common side effects include a dry mouth, large pupils, urinary retention, constipation, and a fast heart rate. It should generally not be used in people with angle closure glaucoma. While there is no evidence that its use during pregnancy causes birth defects, that has not been well studied. It is likely safe during breastfeeding. It is an antimuscarinic (a type of anticholinergic) that works by inhibiting the parasympathetic nervous system. Atropine occurs n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |