|
Methyl Mercaptan
Methanethiol (also known as methyl mercaptan) is an organosulfur compound with the chemical formula . It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals (including humans), as well as in plant tissues. It also occurs naturally in certain foods, such as some nuts and cheese. It is one of the chemical compounds responsible for bad breath and the smell of flatus. Methanethiol is the simplest thiol and is sometimes abbreviated as MeSH. It is very flammable. Structure and reactions The molecule is tetrahedral at the carbon atom, like methanol. It is a weak acid, with a p''K''a of ~10.4, but is about a hundred thousand times more acidic than methanol. The colorless salt can be obtained in this way: :CH3SH + CH3ONa → CH3SNa + CH3OH The resulting thiolate anion is a strong nucleophile. It can be oxidized to dimethyl disulfide: :2CH3SH + → CH3SSCH3 + H2O Further oxidation takes the disulfide to two mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanethiol
Ethanethiol, commonly known as ethyl mercaptan, is an organosulfur compound with the formula CH3CH2SH. is a colorless liquid with a distinct odor. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic in high concentrations. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful. Preparation Ethanethiol is prepared by the reaction of ethene with hydrogen sulfide over a catalyst. The various producers utilize different catalysts in this process. It has also been prepared commercially by the reaction of ethanol with hydrogen sulfide gas over an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and ''electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''cationoid' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marsh
A marsh is a wetland that is dominated by herbaceous rather than woody plant species.Keddy, P.A. 2010. Wetland Ecology: Principles and Conservation (2nd edition). Cambridge University Press, Cambridge, UK. 497 p Marshes can often be found at the edges of lakes and streams, where they form a transition between the aquatic and terrestrial ecosystems. They are often dominated by grasses, rushes or reeds. If woody plants are present they tend to be low-growing shrubs, and the marsh is sometimes called a carr. This form of vegetation is what differentiates marshes from other types of wetland such as swamps, which are dominated by trees, and mires, which are wetlands that have accumulated deposits of acidic peat. Marshes provide habitats for many kinds of invertebrates, fish, amphibians, waterfowl and aquatic mammals. This biological productivity means that marshes contain 0.1% of global sequestered terrestrial carbon. Moreover, they have an outsized influence on climate resi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Sulfide
Sodium sulfide is a chemical compound with the formula Na2 S, or more commonly its hydrate Na2S·9 H2O. Both the anhydrous and the hydrated salts in pure crystalline form are colorless solids, although technical grades of sodium sulfide are generally yellow to brick red owing to the presence of polysulfides and commonly supplied as a crystalline mass, in flake form, or as a fused solid. They are water-soluble, giving strongly alkaline solutions. When exposed to moist air, Na2S and its hydrates emit hydrogen sulfide, an extremely toxic, flammable and corrosive gas which smells like rotten eggs. Some commercial samples are specified as Na2S·''x''H2O, where a weight percentage of Na2S is specified. Commonly available grades have around 60% Na2S by weight, which means that ''x'' is around 3. These grades of sodium sulfide are often marketed as 'sodium sulfide flakes'. Structure Na2S adopts the antifluorite structure, which means that the Na+ centers occupy sites of the fluoride in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recovery Boiler
Recovery boiler is the part of kraft process of pulping where chemicals for white liquor are recovered and reformed from black liquor, which contains lignin from previously processed wood. The black liquor is burned, generating heat, which is usually used in the process of making electricity, much as in a conventional steam power plant. The invention of the recovery boiler by G.H. Tomlinson in the early 1930s was a milestone in the advancement of the kraft process. Recovery boilers are also used in the (less common) sulfite process of wood pulping; this article deals only with recovery boiler use in the kraft process. Function of recovery boilers Concentrated black liquor contains organic dissolved wood residue in addition to sodium sulfate from the cooking chemicals added at the digester. Combustion of the organic portion of chemicals produces heat. In the recovery boiler, heat is used to produce high pressure steam, which is used to generate electricity in a turbine. The turb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol. Occurrence and production DMS originates primarily from DMSP, a major secondary metabolite in some marine algae. DMS is the most abundant biological sulfur compound emitted to the atmosphere. Emission occurs over the oceans by phytoplankton. DMS is also produced naturally by bacterial transformation of dimethyl sulfoxide (DMSO) waste that is disposed of into sewers, where it can cause environmental odor problems. DMS is oxidized in the marine atmos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Methanethiolate
Sodium methanethiolate or sodium thiomethoxide (CH3SNa, MeSNa) is the sodium conjugate base of methanethiol. This compound is commercially available as a white solid. It is a powerful nucleophile that can be used to prepare thioether, methylthioethers. Its hydrolysis in moist air produces methanethiol, which has a low odor threshold and a noxious fecal smell. References Thiolates Organic sodium salts {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolate
Phenolates (also called phenoxides) are anions, salts, and esters of phenols. They may be formed by reaction of phenols with strong base. Properties Alkali metal phenolates, such as sodium phenolate hydrolyze in aqueous solution to form basic solutions. At pH = 10, phenol and phenolate are in approximately 1:1 proportions. Phenolate anions are enolates. As such, they react as nucleophiles at both oxygen and carbon positions. In general, reaction at oxygen occurs under kinetic control, whereas reaction at carbon occurs under thermodynamic control. Uses Alkyl aryl ethers can be synthesized through the Williamson ether synthesis by treating sodium phenolate with an alkyl halide: :C6H5ONa + CH3I → C6H5OCH3 + NaI :C6H5ONa + (CH3O)2SO2 → C6H5OCH3 + (CH3O)SO3Na Production of salicylic acid Salicylic acid is produced in the Kolbe–Schmitt reaction between carbon dioxide and sodium phenolate. : See also * Sodium phenolate Sodium phenoxide (sodium phenolate) is an organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors. History Lignin was first mentioned in 1813 by the Swiss botanist A. P. de Candolle, who described it as a fibrous, tasteless material, insoluble in water and alcohol but soluble in weak alkaline solutions, and which can be precipitated from solution using acid. He named the substance “lignine”, which is derived from the Latin word '' lignum'', meaning wood. It is one of the most abundant organic polymers on Earth, exceeded only by cellulose. Lignin constitutes 30% of non-fossil organic carbon on Earth, and 20 to 35% of the dry mass of wood. Lignin is present in red algae, which suggest that the common ancestor of plants and red algae ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
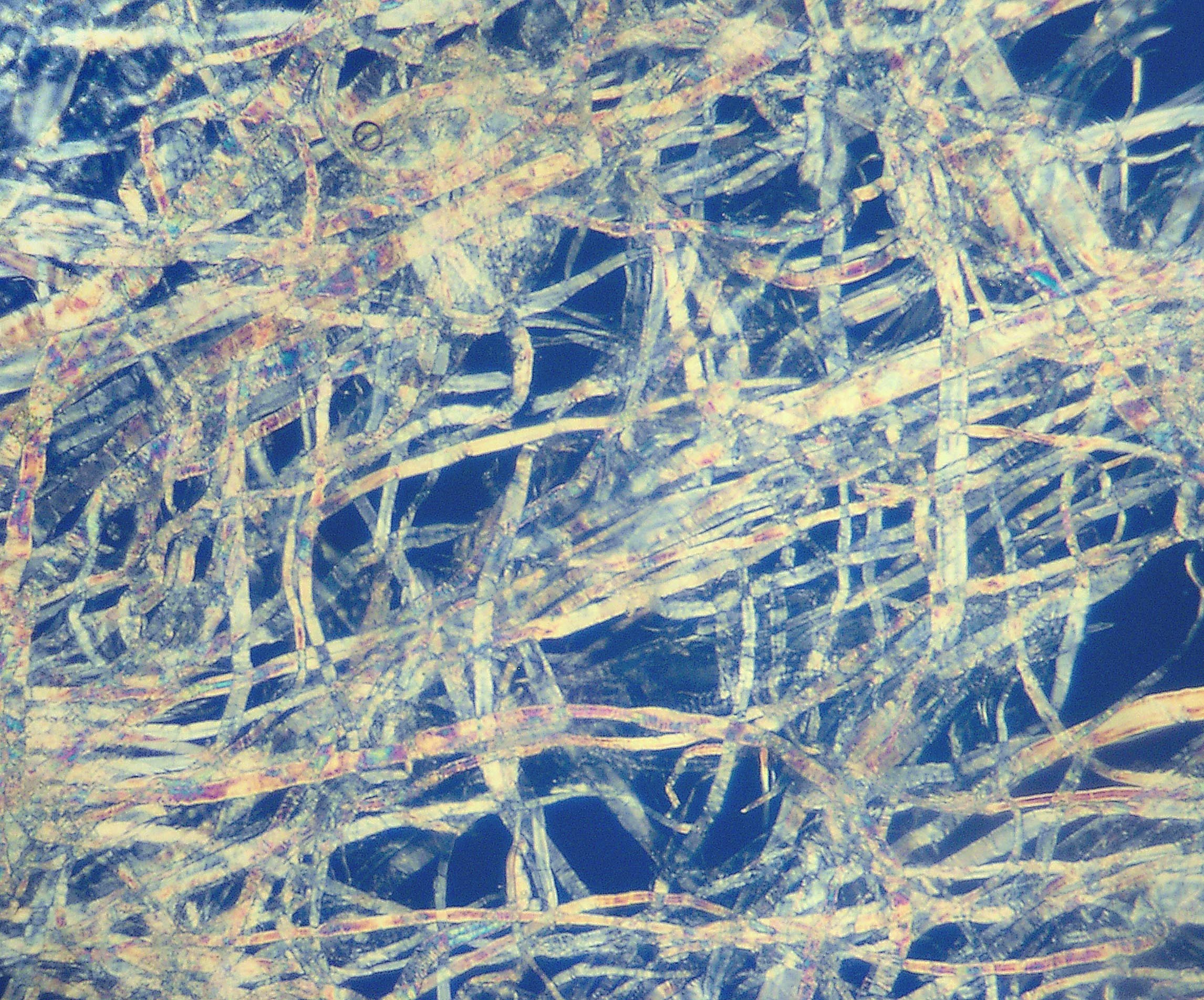
Pulp (paper)
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibers from wood, fiber crops, waste paper, or rags. Mixed with water and other chemical or plant-based additives, pulp is the major raw material used in papermaking and the industrial production of other paper products. History Before the widely acknowledged invention of papermaking by Cai Lun in China around 105 AD, paper-like writing materials such as papyrus and amate were produced by ancient civilizations using plant materials which were largely unprocessed. Strips of bark or bast material were woven together, beaten into rough sheets, dried, and polished by hand. Pulp used in modern and traditional papermaking is distinguished by the process which produces a finer, more regular slurry of cellulose fibers which are pulled out of solution by a screen and dried to form sheets or rolls. The earliest paper produced in China consisted of bast fibers from the paper mulberr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kraft Pulping
The kraft process (also known as kraft pulping or sulfate process) is a process for conversion of wood into wood pulp, which consists of almost pure cellulose fibres, the main component of paper. The kraft process involves treatment of wood chips with a hot mixture of water, sodium hydroxide (NaOH), and sodium sulfide (Na2S), known as white liquor, that breaks the bonds that link lignin, hemicellulose, and cellulose. The technology entails several steps, both mechanical and chemical. It is the dominant method for producing paper. In some situations, the process has been controversial because kraft plants can release odorous products and in some situations produce substantial liquid wastes. The process name is derived from German word ''Kraft'', meaning "strength" in this context, due to the strength of the kraft paper produced using this process. History A precursor of the Kraft process was used during the Napoleonic Wars in England. The kraft process (so called because of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bleach
Bleach is the generic name for any chemical product that is used industrially or domestically to remove color (whitening) from a fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically, to a dilute solution of sodium hypochlorite, also called "liquid bleach". Many bleaches have broad spectrum bactericidal properties, making them useful for disinfecting and sterilizing. They are used in swimming pool sanitation to control bacteria, viruses, and algae, and in many places where sterile conditions are required. They are also used in many industrial processes, notably in the bleaching of wood pulp. Bleaches also have other minor uses like removing mildew, killing weeds, and increasing the longevity of cut flowers. Bleaches work by reacting with many colored organic compounds, such as natural pigments, and turning them into colorless ones. While most bleaches are oxidizing agents (chemicals that can remove electrons from other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |