|
Metaxalone
Metaxalone, sold under the brand name Skelaxin, is a muscle relaxant medication used to relax muscles and relieve pain caused by strains, sprains, and other musculoskeletal conditions. Its exact mechanism of action is not known, but it may be due to general central nervous system depression. It is a moderately strong muscle relaxant, with relatively low incidence of side effects. Common side effects include nausea, vomiting, drowsiness, and central nervous system (CNS) side effects, such as dizziness, headache, and irritability. The metabolism of metaxalone involves enzymes CYP1A2 and CYP2C19 in the cytochrome P450 system. Because many medications are metabolized by enzymes in this system, precaution must be taken when administering it with other medications involving the P450 system to avoid interactions. Because of the potential for side effects, this drug is considered a high risk in the elderly. Pharmacokinetics Metaxalone exhibits increased bioavailability when taken wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscle Relaxants
A muscle relaxant is a drug that affects skeletal muscle function and decreases the muscle tone. It may be used to alleviate symptoms such as muscle spasms, pain, and hyperreflexia. The term "muscle relaxant" is used to refer to two major therapeutic groups: neuromuscular blockers and spasmolytics. Neuromuscular blockers act by interfering with transmission at the neuromuscular end plate and have no central nervous system (CNS) activity. They are often used during surgical procedures and in intensive care and emergency medicine to cause temporary paralysis. Spasmolytics, also known as "centrally acting" muscle relaxant, are used to alleviate musculoskeletal pain and spasms and to reduce spasticity in a variety of neurological conditions. While both neuromuscular blockers and spasmolytics are often grouped together as muscle relaxant, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscle Relaxant
A muscle relaxant is a drug that affects skeletal muscle function and decreases the muscle tone. It may be used to alleviate symptoms such as muscle spasms, pain, and hyperreflexia. The term "muscle relaxant" is used to refer to two major therapeutic groups: neuromuscular blockers and spasmolytics. Neuromuscular blockers act by interfering with transmission at the neuromuscular end plate and have no central nervous system (CNS) activity. They are often used during surgical procedures and in intensive care and emergency medicine to cause temporary paralysis. Spasmolytics, also known as "centrally acting" muscle relaxant, are used to alleviate musculoskeletal pain and spasms and to reduce spasticity in a variety of neurological conditions. While both neuromuscular blockers and spasmolytics are often grouped together as muscle relaxant, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2C9
Cytochrome P450 family 2 subfamily C member 9 (abbreviated CYP2C9) is an enzyme protein. The enzyme is involved in metabolism, by oxidation, of both xenobiotics, including drugs, and endogenous compounds, including fatty acids. In humans, the protein is encoded by the ''CYP2C9'' gene. The gene is highly polymorphic, which affects the efficiency of the metabolism by the enzyme. Function CYP2C9 is a crucial cytochrome P450 enzyme, which plays a significant role in the metabolism, by oxidation, of both xenobiotic and endogenous compounds. CYP2C9 makes up about 18% of the cytochrome P450 protein in liver microsomes. The protein is mainly expressed in liver, duodenum and small intestine. About 100 therapeutic drugs are metabolized by CYP2C9, including drugs with a narrow therapeutic index such as warfarin and phenytoin, and other routinely prescribed drugs such as acenocoumarol, tolbutamide, losartan, glipizide, and some nonsteroidal anti-inflammatory drugs. By contrast, the known extr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pfizer Brands
Pfizer Inc. ( ) is an American multinational pharmaceutical and biotechnology corporation headquartered on 42nd Street in Manhattan, New York City. The company was established in 1849 in New York by two German entrepreneurs, Charles Pfizer (1824–1906) and his cousin Charles F. Erhart (1821–1891). Pfizer develops and produces medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology. The company has several blockbuster drugs or products that each generate more than billion in annual revenues. In 2020, 52% of the company's revenues came from the United States, 6% came from each of China and Japan, and 36% came from other countries. Pfizer was a component of the Dow Jones Industrial Average stock market index from 2004 to August 2020. The company ranks 64th on the Fortune 500 and 49th on the Forbes Global 2000. History 1849–1950: Early history Pfizer was founded in 1849 by Charles Pfizer and Charles F. Erhart, two cousins who had im ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
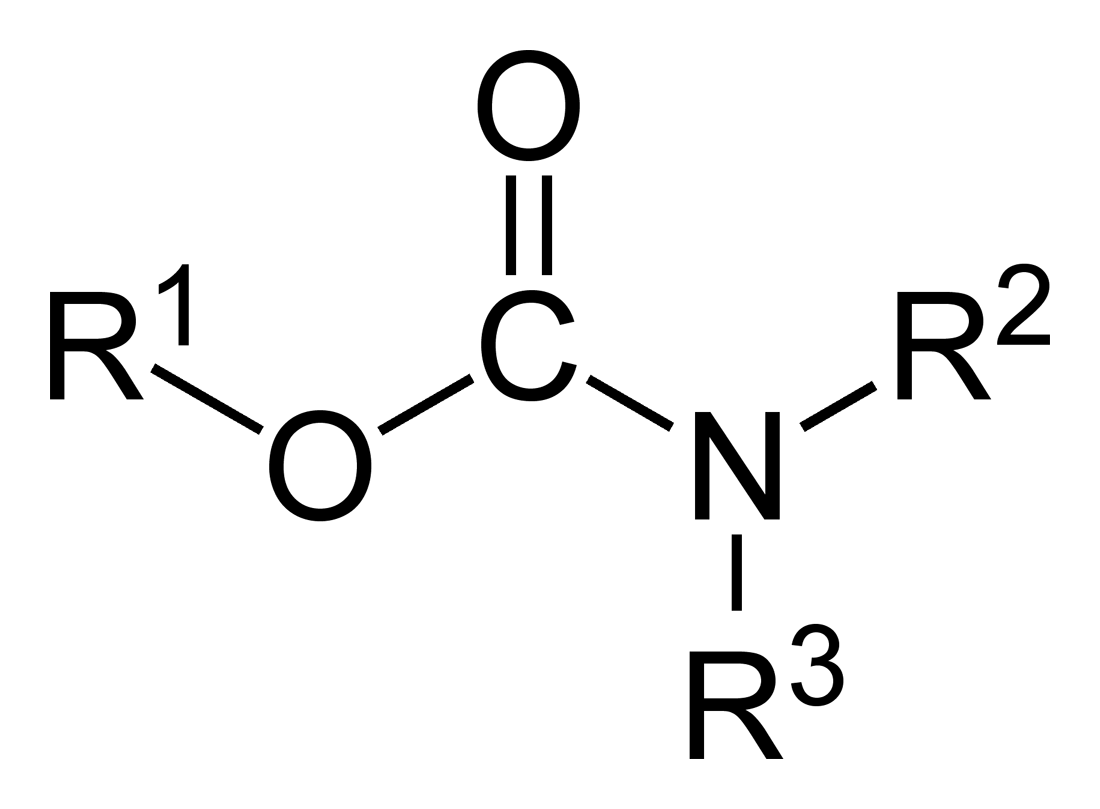
Carbamates
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate immediately, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs which are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will therefore either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprotein, i.e. a protein containing a heme group with an iron atom. In humans, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A
Cytochrome P450, family 3, subfamily A, also known as CYP3A, is a human gene locus. A homologous locus is found in mice. The CYP3A locus includes all the known members of the 3A subfamily of the cytochrome P450 superfamily of genes. These genes encode monooxygenases which catalyze many reactions involved in drug metabolism and synthesis of cholesterol, steroids and other lipids. The CYP3A cluster consists of four genes: * CYP3A4, * CYP3A5, * CYP3A7, and * CYP3A43 Cytochrome P450 3A43 is a protein that in humans is encoded by the ''CYP3A43'' gene. This gene encodes a member of the cytochrome P450 superfamily of enzymes. The cytochrome P450 proteins are monooxygenases which catalyze many reactions involved .... The region also contains four pseudogenes: * , * , * , and * . as well as several extra exons which may or may not be included in transcripts produced from this region. Previously another CYP3A member, CYP3A3, was thought to exist; however, it is now thought that thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2E1
Cytochrome P450 2E1 (abbreviated CYP2E1, ) is a member of the cytochrome P450 mixed-function oxidase system, which is involved in the metabolism of xenobiotics in the body. This class of enzymes is divided up into a number of subcategories, including CYP1, CYP2, and CYP3, which as a group are largely responsible for the breakdown of foreign compounds in mammals. While CYP2E1 itself carries out a relatively low number of these reactions (~4% of known P450-mediated drug oxidations), it and related enzymes CYP1A2 and CYP3A4 are responsible for the breakdown of many toxic environmental chemicals and carcinogens that enter the body, in addition to basic metabolic reactions such as fatty acid oxidations. Function CYP2E1 is a membrane protein expressed in high levels in the liver, where it composes nearly 50% of the total hepatic cytochrome P450 mRNA and 7% of the hepatic cytochrome P450 protein. The liver is therefore where most drugs undergo deactivation by CYP2E1, either directly o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of CYP2D6 enzyme produced betwee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is generally lower than that of intravenous due to intestinal endothelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |