|
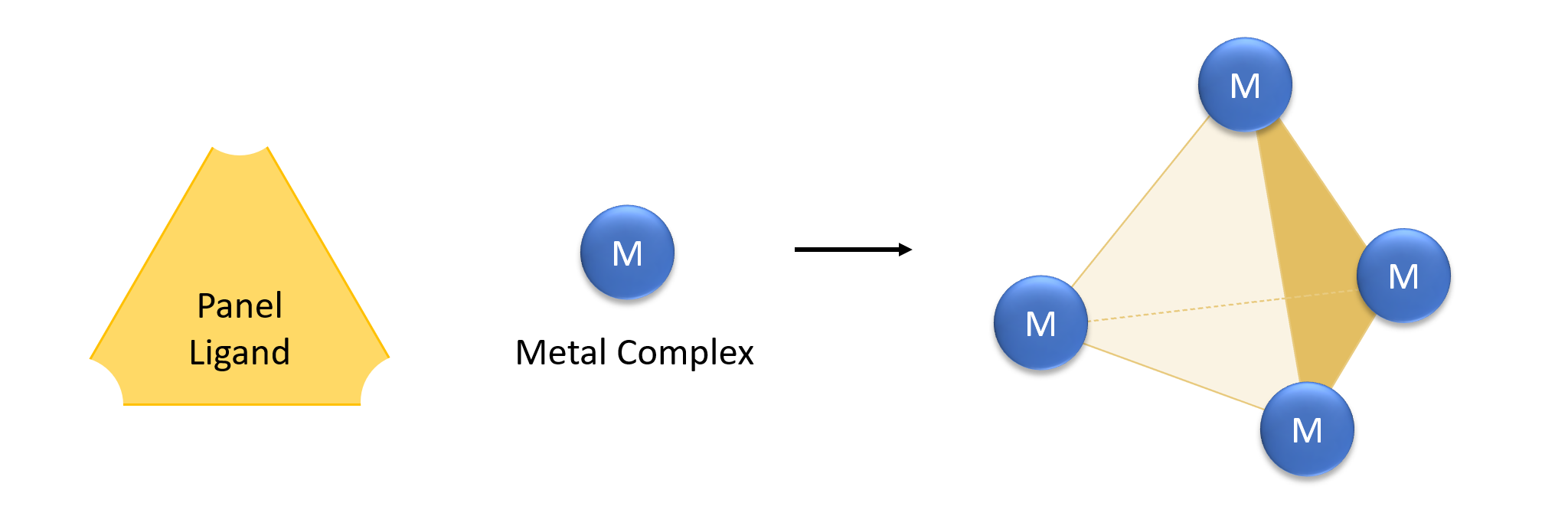
Metallaprism
Coordination cages are three-dimensional ordered structures in solution that act as hosts in host–guest chemistry. They are self-assembled in solution from organometallic precursors, and often rely solely on noncovalent interactions rather than covalent bonds. Coordinate bonds are useful in such supramolecular self-assembly because of their versatile geometries. However, there is controversy over calling coordinate bonds noncovalent, as they are typically strong bonds and have covalent character. The combination of a coordination cage and a guest is a type of inclusion compound. Coordination complexes can be used as "nano-laboratories" for synthesis, and to isolate interesting intermediates. The inclusion complexes of a guest inside a coordination cage show intriguing chemistry as well; often, the properties of the cage will change depending on the guest. Coordination complexes are molecular moieties, so they are distinct from clathrates and metal-organic frameworks. History Chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triazine
Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is . They exist in three isomeric forms, 1,3,5-triazines being common. Structure The triazines have planar six-membered benzene-like ring but with three carbons replaced by nitrogens. The three isomers of triazine are distinguished by the positions of their nitrogen atoms, and are referred to as 1,2,3-triazine, 1,2,4-triazine, and 1,3,5-triazine. Other aromatic nitrogen heterocycles are pyridines with one ring nitrogen atom, diazines with 2 nitrogen atoms in the ring, triazoles with 3 nitrogens in a 5 membered ring, and tetrazines with 4 ring nitrogen atoms. Uses Melamine A well known triazine is melamine (2,4,6-triamino-1,3,5-triazine). With three amino substituents, melamine is a precursor to commercial resins. Guanamines are closely related to melamine, except with one amino substituent replaced by an organic group. This difference is exploited in the use of guanamines t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Cage
Coordination cages are three-dimensional ordered structures in solution that act as hosts in host–guest chemistry. They are self-assembled in solution from organometallic precursors, and often rely solely on noncovalent interactions rather than covalent bonds. Coordinate bonds are useful in such supramolecular self-assembly because of their versatile geometries. However, there is controversy over calling coordinate bonds noncovalent, as they are typically strong bonds and have covalent character. The combination of a coordination cage and a guest is a type of inclusion compound. Coordination complexes can be used as "nano-laboratories" for synthesis, and to isolate interesting intermediates. The inclusion complexes of a guest inside a coordination cage show intriguing chemistry as well; often, the properties of the cage will change depending on the guest. Coordination complexes are molecular moieties, so they are distinct from clathrates and metal-organic frameworks. History Chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Host–guest Chemistry
In supramolecular chemistry, host–guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host–guest chemistry encompasses the idea of molecular recognition and interactions through non-covalent bonding. Non-covalent bonding is critical in maintaining the 3D structure of large molecules, such as proteins and is involved in many biological processes in which large molecules bind specifically but transiently to one another. Although non-covalent interactions could be roughly divided into those with more electrostatic or dispersive contributions, there are few commonly mentioned types of non-covalent interactions: ionic bonding, hydrogen bonding, van der Waals forces and hydrophobic interactions. Overview Host–guest chemistry is a branch of supramolecular chemistry in which a host molecule forms a chemical compound with a guest molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal Complexes
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Derivative (chemistry)
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction. In the past, derivative also meant a compound that ''can be imagined to'' arise from another compound, if one atom or group of atoms is replaced with another atom or group of atoms, but modern chemical language now uses the term structural analog for this meaning, thus eliminating ambiguity. The term "structural analogue" is common in organic chemistry. In biochemistry, the word is used for compounds that at least theoretically can be formed from the precursor compound. Chemical derivatives may be used to facilitate analysis. For example, melting point (MP) analysis can assist in identification of many organic compounds. A crystalline derivative may be prepared, such as a semicarbazone or 2,4-dinitrophenylhydrazone (derived from aldehydes or ketones), as a simple way of verifying the identity of the original compound, assuming that a table of derivative MP values is available ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioconjugate
Bioconjugation is a chemical strategy to form a stable Covalent bond, covalent link between two molecules, at least one of which is a biomolecule. Function Recent advances in the understanding of biomolecules enabled their application to numerous fields like medicine and materials. Synthetically modified biomolecules can have diverse functionalities, such as tracking cellular events, revealing enzyme function, determining protein biodistribution, Imaging science, imaging specific biomarkers, and delivering Pharmaceutical drug, drugs to targeted cells. Bioconjugation is a crucial strategy that links these modified biomolecules with different substrate (biochemistry), substrates. Synthesis Chemical synthesis, Synthesis of bioconjugates involves a variety of challenges, ranging from the simple and nonspecific use of a fluorescent dye marker to the complex design of Antibody-drug conjugate, antibody drug conjugates. As a result, various bioconjugation reactions – chemical reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cymene
''p''-Cymene is a naturally occurring aromatic organic compound. It is classified as an alkylbenzene related to a monoterpene. Its structure consists of a benzene ring ''para''-substituted with a methyl group and an isopropyl group. ''p''-Cymene is insoluble in water, but miscible with organic solvents. Isomers and production In addition to ''p''-cymene, two less common geometric isomers are ''o''-cymene, in which the alkyl groups are ''ortho''-substituted, and ''m''-cymene, in which they are ''meta''-substituted. ''p''-Cymene is the only natural isomer, as expected from the terpene rule. All three isomers form the group of cymenes. Cymene is also produced by alkylation of toluene with propylene. Related compounds It is a constituent of a number of essential oils, most commonly the oil of cumin and thyme. Significant amounts are formed in sulfite pulping process from the wood terpenes. ''p''-Cymene is a common ligand for ruthenium. The parent compound is 2.html" ;"tit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the liquid phase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inclusion Compound
In host–guest chemistry, an inclusion compound (also known as an inclusion complex) is a chemical complex in which one chemical compound (the "host") has a cavity into which a "guest" compound can be accommodated. The interaction between the host and guest involves purely van der Waals bonding. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which guest molecules can fit. Examples and case studies Calixarenes Calixarenes and related formaldehyde-arene condensates are one class of hosts that form inclusion compounds. One famous illustration is the adduct with cyclobutadiene, which otherwise is unstable. Cyclodextrins Cyclodextrins are well established hosts for the formation of inclusion compounds. Illustrative is the case of ferrocene which is inserted into the cyclodextrin at 100 °C under hydrothermal conditions. Cyclodextrin also forms inclusion compounds with fragrances. As a result, the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |