|
Mesylate
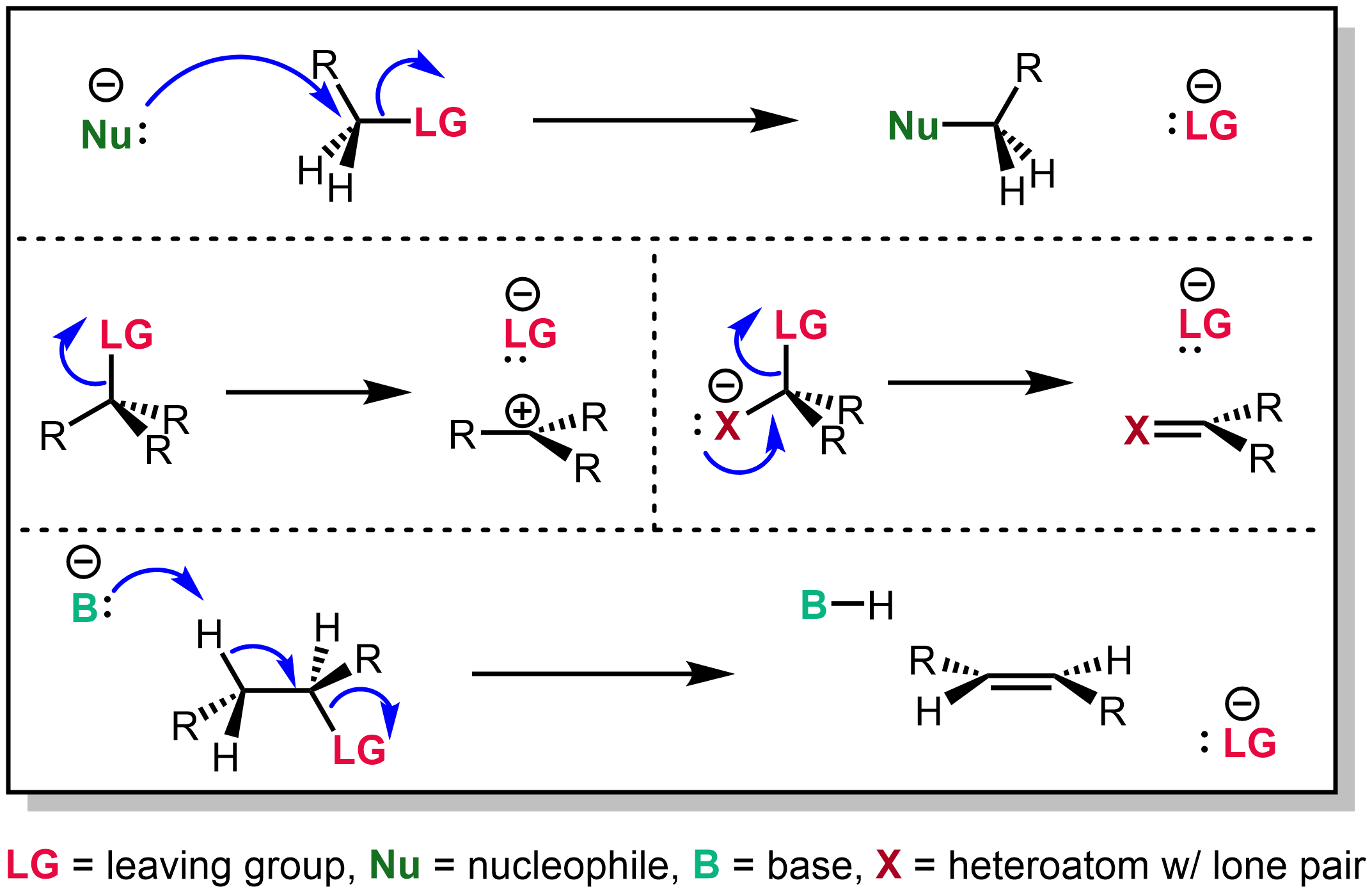
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group or anion, the spelling used is sometimes mesilate (as in ''imatinib mesilate'', the mesylate salt of imatinib). Mesylate esters are a group of organic compounds that share a common functional group with the general structure , abbreviated , where R is an organic substituent. Mesylate is considered a leaving group in nucleophilic substitution reactions. Preparation Mesylates are generally prepared by treating an alcohol and methanesulfonyl chloride in the presence of a base, such as triethylamine. Mesyl Related to mesylate is the mesyl (Ms) or methanesulfonyl (CH3SO2) functional group. Methanesulfonyl chloride is often referred to as mesyl chloride. Whereas mesylates are often hydrolytically labile, mesyl groups, when attached to nitroge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imatinib
Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral chemotherapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple receptor tyrosine kinases such as CSF1R, ABL, c-KIT, FLT3, and PDGFR-β. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome-positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome. Common side effects include vomiting, diarrhea, muscle pain, headache, and rash. Severe side effects may include fluid retention, gastrointestinal bleeding, bone marrow suppression, liver problems, and heart failure. Use during pregnancy may result in harm to the baby. Imatinib works by stopping the Bcr-Abl tyrosine-kinase. This can slow growth or result in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mesylate Anion Structural Formulae
In organosulfur chemistry, a mesylate is any salt or ester of methanesulfonic acid (). In salts, the mesylate is present as the anion. When modifying the international nonproprietary name of a pharmaceutical substance containing the group or anion, the spelling used is sometimes mesilate (as in ''imatinib mesilate'', the mesylate salt of imatinib). Mesylate esters are a group of organic compounds that share a common functional group with the general structure , abbreviated , where R is an organic substituent. Mesylate is considered a leaving group in nucleophilic substitution reactions. Preparation Mesylates are generally prepared by treating an alcohol and methanesulfonyl chloride in the presence of a base, such as triethylamine. Mesyl Related to mesylate is the mesyl (Ms) or methanesulfonyl (CH3SO2) functional group. Methanesulfonyl chloride is often referred to as mesyl chloride. Whereas mesylates are often hydrolytically labile, mesyl groups, when attached to nitro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanesulfonic Acid
Methanesulfonic acid (MsOH) or methanesulphonic acid (in British English) is an organosulfuric, colorless liquid with the chemical formula and structure . It is the simplest of the alkylsulfonic acids (). Salts and esters of methanesulfonic acid are known as mesylates (or methanesulfonates, as in ethyl methanesulfonate). It is hygroscopic in its concentrated form. Methanesulfonic acid can dissolve a wide range of metal salts, many of them in significantly higher concentrations than in hydrochloric acid (HCl) or sulfuric acid (). Applications Methanesulfonic acid is used as an acid catalyst in organic reactions because it is a non-volatile, strong acid that is soluble in organic solvents. It is convenient for industrial applications because it is liquid at ambient temperature, while the closely related ''p''-toluenesulfonic acid (PTSA) is solid. However, in a laboratory setting, solid PTSA is more convenient. Methanesulfonic acid can be used in the generation of borane (BH3) b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many indus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiac
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest. In humans, other mammals, and birds, the heart is divided into four chambers: upper left and right atria and lower left and right ventricles. Commonly the right atrium and ventricle are referred together as the right heart and their left counterparts as the left heart. Fish, in contrast, have two chambers, an atrium and a ventricle, while most reptiles have three chambers. In a healthy heart blood flows one way through the heart due to heart valves, which prevent backflow. The heart is enclosed in a protective sac, the pericardium, which also contains a small amount of fluid. The wall o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiarrhythmic
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormally fast rhythms ( tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular tachycardia. Many attempts have been made to classify antiarrhythmic agents. Many of the antiarrhythmic agents have multiple modes of action, which makes any classification imprecise. Vaughan Williams classification The Vaughan Williams classification was introduced in 1970 by Miles Vaughan Williams.Vaughan Williams, EM (1970) "Classification of antiarrhythmic drugs". In ''Symposium on Cardiac Arrhythmias'' (Eds. Sandoe E; Flensted-Jensen E; Olsen KH). Astra, Elsinore. Denmark (1970) Vaughan Williams was a pharmacology tutor at Hertford College, Oxford. One of his students, Bramah N. Singh, contributed to the development of the classification system. The system is therefore sometimes known as the Singh-Vaughan Williams classification. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
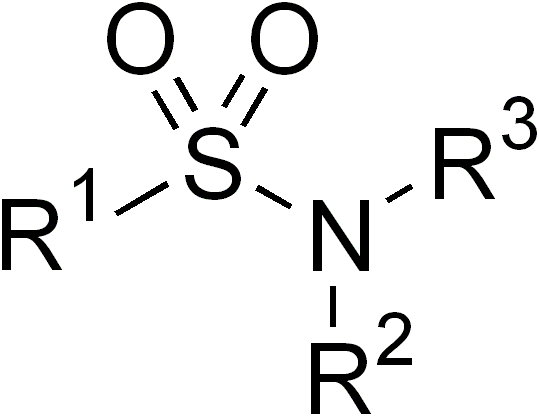
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sotalol
Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. It is only recommended in those with significant abnormal heart rhythms due to potentially serious side effects. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or injection into a vein. Common side effects include a slow heart rate, chest pain, low blood pressure, feeling tired, dizziness, shortness of breath, problems seeing, vomiting, and swelling. Other serious side effects may include QT prolongation, heart failure, or bronchospasm. Sotalol is a non-selective beta-adrenergic receptor blocker which has both class II and class III antiarrhythmic properties. Sotalol was first described in 1964 and came into medical use in 1974. It is available as a generic medication. In 2020, it was the 296th most commonly prescribed medication in the United States, with more than 1million prescriptions. Medical uses Acc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ibutilide
Ibutilide is a Class III antiarrhythmic agent that is indicated for acute cardioconversion of atrial fibrillation and atrial flutter of a recent onset to sinus rhythm. It exerts its antiarrhythmic effect by induction of slow inward sodium current, which prolongs action potential and refractory period (physiology) of myocardial cells. Because of its Class III antiarrhythmic activity, there should not be concomitant administration of Class Ia and Class III agents. Ibutilide is marketed as Corvert by Pfizer. Administration resulted in successful heart rhythm control in 31-44% of patients within 90 minutes, with sustained polymorphic ventricular tachycardia in 0.9-2.5% of patients. It appears to show better results in atrial flutter as compared to atrial fibrillation. Mechanism of action Ibutilide, like other class III antiarrhythmic drugs, blocks delayed rectified potassium current. It does have action on the slow sodium channel and promotes the influx of sodium through these slo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanesulfonyl Chloride
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula . Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group –, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).Valerie Vaillancourt, Michele M. Cudahy, Matthew M. Kreilein and Danielle L. Jacobs "Methanesulfonyl Chloride" in E-EROS Encyclopedia for Reagents in Organic Synthesis. Preparation It is manufactured by the reaction of methane and sulfuryl chloride in a radical reaction: : Another method of manufacture entails chlorination of methanesulfonic acid with thionyl chloride or phosgene: : : Reactions Methanesulfonyl chloride is a precursor to many compounds because it is high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sematilide
Sematilide is an antiarrhythmic agent Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormally fast rhythms ( tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular ta .... Sulfonamides Antiarrhythmic agents Benzamides Diethylamino compounds {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |