|
Merrilactone A
Merrilactone A is one of the four sesquiterpenes that were newly discovered from the fruit of ''Illicium merrillianum'' in 2000. Members of the genus ''Illicium'' include Chinese star anise, widely used as a spice for flavouring food and beverages, and also poisonous plants such as Japanese star anise. Chemical studies of ''Illicium'' have developed rapidly over the last 20 years, and merrilactone A has been shown to have neurotrophic activity in fetal rat Cerebral cortex, cortical neuron cultures. This has led researchers to believe that Merrilactone A may hold therapeutic potential in the treatment of neuro-degenerative diseases such as Alzheimer's disease and Parkinson's disease. Occurrence Merrilactone A occurs naturally in ''Illicium merrillianum'', a plant indigenous to southern China and Myanmar. The genus ''Illicium'' belongs to the family ''Illiciaceae'' and is an evergreen shrub or tree. Approximately 40 species are disjunctively distributed in eastern North America, Mex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. Sesquiterpenes are found naturally in plants and insects, as semiochemicals, e.g. defensive agents or pheromones. Biosynthesis and examples The reaction of geranyl pyrophosphate with isopentenyl pyrophosphate results in the 15-carbon farnesyl pyrophosphate (FPP), which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Cyclic sesquiterpenes are more common than cyclic monoterpenes because of the increased chain length and additional double bond in the sesquiterpene precursors. In addition to common six-membered ring systems such as the ones found in zingiberene and bisacurone, cyclization of one end of the chain to the other end can l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Follicle (fruit)
In botany, a follicle is a dry unilocular fruit formed from one carpel, containing two or more seeds. It is usually defined as dehiscing by a suture in order to release seeds, for example in ''Consolida'' (some of the larkspurs), peony and milkweed (''Asclepias''). Some difficult cases exist however, so that the term indehiscent follicle is sometimes used, for example with the genus ''Filipendula'', which has indehiscent fruits that could be considered intermediate between a (dehiscent) follicle and an (indehiscent) achene. An aggregate fruit that consists of follicles may be called a follicetum. Examples include hellebore, aconite, ''Delphinium'', ''Aquilegia'' or the family Crassulaceae, where several follicles occur in a whorl on a shortened receptacle, or ''Magnolia'', which has many follicles arranged in a spiral on an elongated receptacle. The follicles of some species dehisce by the ventral suture (as in ''Banksia''), or by the dorsal suture (as in ''Magnolia'').Kapil, R. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Farnesyl Diphosphate
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for ''N''-glycosylation). Biosynthesis Farnesyl pyrophosphate synthase (a prenyl transferase) catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate, as is shown in the following two steps: * Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrophosphate: * Geranyl pyrophosphate then reacts with another molecule of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate Pharmacology The above reactions are inhibited by bisphosphonates (used for osteoporosis). Farnesyl pyrophosphate is a selec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopentenyl Diphosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria parasites, and in the plastids of higher pl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylallyl Diphosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP. In the mevalonate pathway DMAPP is synthesised from mevalonic acid. In contrast, DMAPP is synthesised from HMBPP in the MEP pathway. At present, it is believed that there is crossover between the two pathways in organisms that use both pathways to create terpenes and terpenoid The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes" ...s, such as in plants, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
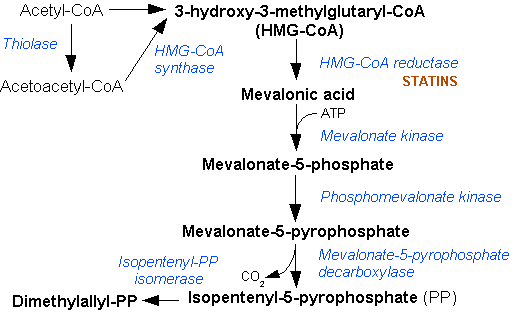
Mevalonic Acid Pathway
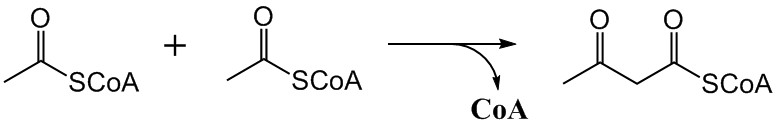
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuroprotection
Neuroprotection refers to the relative preservation of neuronal structure and/or function. In the case of an ongoing insult (a neurodegenerative insult) the relative preservation of neuronal integrity implies a reduction in the rate of neuronal loss over time, which can be expressed as a differential equation. It is a widely explored treatment option for many central nervous system (CNS) disorders including neurodegenerative diseases, stroke, traumatic brain injury, spinal cord injury, and acute management of neurotoxin consumption (i.e. methamphetamine overdoses). Neuroprotection aims to prevent or slow disease progression and secondary injuries by halting or at least slowing the loss of neurons. Despite differences in symptoms or injuries associated with CNS disorders, many of the mechanisms behind neurodegeneration are the same. Common mechanisms of neuronal injury include decreased delivery of oxygen and glucose to the brain, energy failure, increased levels in oxidative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neurite
A neurite or neuronal process refers to any projection from the cell body of a neuron. This projection can be either an axon or a dendrite. The term is frequently used when speaking of immature or developing neurons, especially of cells in culture, because it can be difficult to tell axons from dendrites before differentiation is complete. Neurite development The development of a neurite requires a complex interplay of both extracellular and intracellular signals. At every given point along a developing neurite, there are receptors detecting both positive and negative growth cues from every direction in the surrounding space. The developing neurite sums together all of these growth signals in order to determine which direction the neurite will ultimately grow towards. While not all of the growth signals are known, several have been identified and characterized. Among the known extracellular growth signals are netrin, a midline chemoattractant, and semaphorin, ephrin and collaps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |