|
Menthol (band)
Menthol is an organic compound, more specifically a monoterpenoid, made synthetically or obtained from the oils of corn mint, peppermint, or other mints. It is a waxy, clear or white crystalline substance, which is solid at room temperature and melts slightly above. The main form of menthol occurring in nature is (−)-menthol, which is assigned the (1''R'',2''S'',5''R'') configuration. Menthol has local anesthetic and counterirritant qualities, and it is widely used to relieve minor throat irritation. Menthol also acts as a weak κ-opioid receptor agonist. Structure Natural menthol exists as one pure stereoisomer, nearly always the (1''R'',2''S'',5''R'') form (bottom left corner of the diagram below). The eight possible stereoisomers are: : In the natural compound, the isopropyl group is in the ''trans'' orientation to both the methyl and hydroxyl groups. Thus, it can be drawn in any of the ways shown: : The (+)- and (−)- enantiomers of menthol are the most stable a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Menthol (data Page)
This page provides supplementary chemical data on Menthol. Material Safety Data Sheet The handling of this chemical may incur notable safety precautions. It is highly recommended that you seek the Material Safety Datasheet (MSDS A safety data sheet (SDS), material safety data sheet (MSDS), or product safety data sheet (PSDS) is a document that lists information relating to occupational safety and health for the use of various substances and products. SDSs are a widely ...) for this chemical from a reliable source such aSIRIor the links below, and follow its directions.(''l''-form)(DL or racemic form)(''l''-form)Menthol Eucalyptus ointment Structure and properties Thermodynamic properties Spectral data References {{reflist Chemical data pages Chemical data pages cleanup ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mentha
''Mentha'' (also known as mint, from Greek , Linear B ''mi-ta'') is a genus of plants in the family Lamiaceae (mint family). The exact distinction between species is unclear; it is estimated that 13 to 24 species exist. Hybridization occurs naturally where some species' ranges overlap. Many hybrids and cultivars are known. The genus has a subcosmopolitan distribution across Europe, Africa - (Southern Africa), Asia, Australia - Oceania, North America and South America. Its species can be found in many environments, but most grow best in wet environments and moist soils. Description Mints are aromatic, almost exclusively perennial herbs. They have wide-spreading underground and overground stolons and erect, square, branched stems. Mints will grow 10–120 cm (4–48 inches) tall and can spread over an indeterminate area. Due to their tendency to spread unchecked, some mints are considered invasive. The leaves are arranged in opposite pairs, from oblong to lanceol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
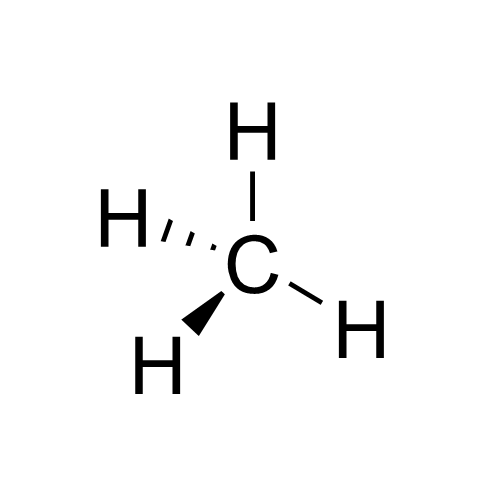
Methyl
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question ( dynamic stereochemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereoisomer
In stereochemistry, stereoisomerism, or spatial isomerism, is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms (constitution), but differ in the three-dimensional orientations of their atoms in space. This contrasts with structural isomers, which share the same molecular formula, but the bond connections or their order differs. By definition, molecules that are stereoisomers of each other represent the same structural isomer. Enantiomers Enantiomers, also known as optical isomers, are two stereoisomers that are related to each other by a reflection: they are mirror images of each other that are non-superposable. Human hands are a macroscopic analog of this. Every stereogenic center in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different optical isom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
κ-opioid Receptor
The κ-opioid receptor or kappa opioid receptor, abbreviated KOR or KOP, is a G protein-coupled receptor that in humans is encoded by the ''OPRK1'' gene. The KOR is coupled to the G protein Gi/G0 and is one of four related receptors that bind opioid-like compounds in the brain and are responsible for mediating the effects of these compounds. These effects include altering nociception, consciousness, motor control, and mood. Dysregulation of this receptor system has been implicated in alcohol and drug addiction. The KOR is a type of opioid receptor that binds the opioid peptide dynorphin as the primary endogenous ligand (substrate naturally occurring in the body). In addition to dynorphin, a variety of natural alkaloids, terpenes and synthetic ligands bind to the receptor. The KOR may provide a natural addiction control mechanism, and therefore, drugs that target this receptor may have therapeutic potential in the treatment of addiction. There is evidence that distribution ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Throat Irritation
Throat irritation can refer to a dry cough, a scratchy feeling at the back of the throat, a sensation of a lumpy feeling, something stuck at the back of the throat, or possibly a feeling of dust in the throat. The symptoms are unpleasant and usually temporary, but occasionally signifies a more serious health issue, such as laryngitis. Common cold COVID 19 Allergy During the summer months, allergies are a common cause of throat irritation. Many individuals have allergies to pet dander, dust, mites, pollen and molds that can trigger an allergic reaction which present with Rhinorrhea, runny nose, red eyes, congested nose and throat irritation. Often a dry cough may also be present. Laryngitis It is inflammation of the larynx, voice box which can occur from overuse, irritation or infection. Laryngitis can be a short term illness or a prolonged problem. The majority of cases of laryngitis are due to virus, viral infections that only last a few days. Laryngitis is often a common co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Counterirritant
A counterirritant is a substance which creates irritation or mild inflammation in one location with the goal of lessening discomfort and/or inflammation in another location. This strategy falls into the more general category of counterstimulation. Topical counter-irritants are non-analgesic, non-anesthetic substances or treatments used to treat pain. Capsaicin, menthol (mint oil), methyl salicylate, and camphor are examples of counterirritants. Heat and cold therapy and massage relieve pain by counterstimulation. The US Food and Drug Administration defines a counterirritant as "An externally applied substance that causes irritation or mild inflammation of the skin for the purpose of relieving pain in muscles, joints and viscera distal to the site of application. They differ from the anesthetics, analgesics, and antipruritic agents, however, in that the pain relief they produce results from stimulation—rather than depression—of the cutaneous sensory receptors and occurs in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Local Anesthetic
A local anesthetic (LA) is a medication that causes absence of pain sensation. In the context of surgery, a local anesthetic creates an absence of pain in a specific location of the body without a loss of consciousness, as opposed to a general anesthetic. When it is used on specific nerve pathways (local anesthetic nerve block), paralysis (loss of muscle power) also can be achieved. Examples Short Duration & Low Potency Procaine Chloroprocaine Medium Duration & Potency Lidocaine Prilocaine High Duration & Potency Tetracaine Bupivacaine Cinchocaine Ropivacaine Clinical LAs belong to one of two classes: aminoamide and aminoester local anesthetics. Synthetic LAs are structurally related to cocaine. They differ from cocaine mainly in that they have a very low abuse potential and do not produce hypertension or (with few exceptions) vasoconstriction. They are used in various techniques of local anesthesia such as: * Topical anesthesia (surface) * Topical administration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |