|
Medical Dictionary For Regulatory Activities
A subscription-based product of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), MedDRA or Medical Dictionary for Regulatory Activities is a clinically validated international medical terminology dictionary-thesaurus used by regulatory authorities and the biopharmaceutical industry during the regulatory process, from pre-marketing (clinical research phase 0 to phase 3) to post-marketing activities (pharmacovigilance or clinical research phase 4), and for safety information data entry, retrieval, evaluation, and presentation. Also, it is the adverse event classification dictionary. The first version of MedDRA was released in 1999 in English and Japanese. MedDRA is now translated into Chinese, Czech, Dutch, French, German, Hungarian, Italian, Korean, Portuguese, Brazilian Portuguese, Russian, and Spanish. In MedDRA version 25.0, Swedish and Latvian translations were also added. In many countries/regions the use of MedD ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Council For Harmonisation Of Technical Requirements For Pharmaceuticals For Human Use
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is an initiative that brings together regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceutical product development and registration. The mission of the ICH is to promote public health by achieving greater harmonisation through the development of technical Guidelines and requirements for pharmaceutical product registration. Harmonisation leads to a more rational use of human, animal and other resources, the elimination of unnecessary delay in the global development, and availability of new medicines while maintaining safeguards on quality, safety, efficacy, and regulatory obligations to protect public health. Junod notes in her 2005 treatise on Clinical Drug Trials that "Above all, the ICH has succeeded in aligning clinical trial requirements." History In the 1980s the European Union began harmonising regulatory requirem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
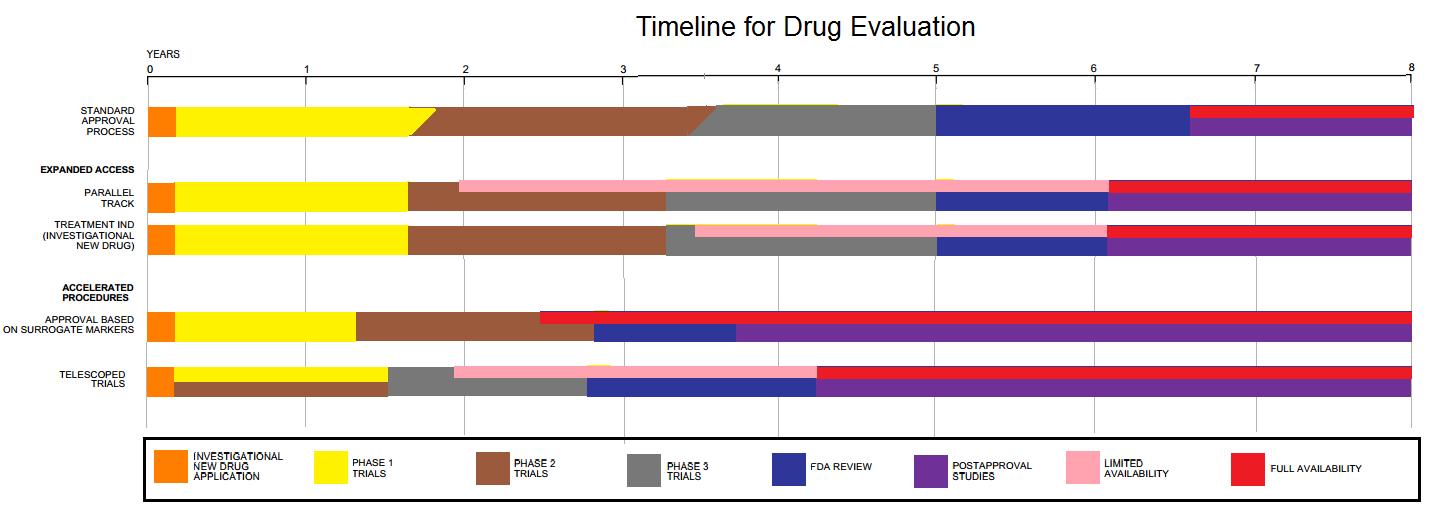
Clinical Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacovigilance
Term Given By Tushar Sharma (UPES Batch 2025) Pharmacovigilance (PV, or PhV), also known as drug safety, is the pharmaceutical science relating to the "collection, detection, assessment, monitoring, and prevention" of adverse effects with pharmaceutical products. The etymological roots for the word "pharmacovigilance" are: (Greek for drug) and (Latin for to keep watch). As such, pharmacovigilance heavily focuses on adverse drug reactions (ADR), which are defined as any response to a drug which is noxious and unintended, including lack of efficacy (the condition that this definition only applies with the doses normally used for the prophylaxis, diagnosis or therapy of disease, or for the modification of physiological disorder function was excluded with the latest amendment of the applicable legislation). Medication errors such as overdose, and misuse and abuse of a drug as well as drug exposure during pregnancy and breastfeeding, are also of interest, even without an adverse ev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trials
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Terminology
Medical terminology is a language used to precisely describe the human body including all its components, processes, conditions affecting it, and procedures performed upon it. Medical terminology is used in the field of medicine Medical terminology has quite regular morphology, the same prefixes and suffixes are used to add meanings to different roots. The root of a term often refers to an organ, tissue, or condition. For example, in the disorder known as hypertension, the prefix "hyper-" means "high" or "over", and the root word "tension" refers to pressure, so the word "hypertension" refers to abnormally high blood pressure. The roots, prefixes and suffixes are often derived from Greek or Latin, and often quite dissimilar from their English-language variants. This regular morphology means that once a reasonable number of morphemes are learnt it becomes easy to understand very precise terms assembled from these morphemes. Much medical language is anatomical terminology, concern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Classification Of Diseases
The International Classification of Diseases (ICD) is a globally used diagnostic tool for epidemiology, health management and clinical purposes. The ICD is maintained by the World Health Organization (WHO), which is the directing and coordinating authority for health within the United Nations System. The ICD is originally designed as a health care classification system, providing a system of diagnostic codes for classifying diseases, including nuanced classifications of a wide variety of signs, symptoms, abnormal findings, complaints, social circumstances, and external causes of injury or disease. This system is designed to map health conditions to corresponding generic categories together with specific variations, assigning for these a designated code, up to six characters long. Thus, major categories are designed to include a set of similar diseases. The ICD is published by the WHO and used worldwide for morbidity and mortality statistics, reimbursement systems, and automated d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicines And Healthcare Products Regulatory Agency
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe. The MHRA was formed in 2003 with the merger of the Medicines Control Agency (MCA) and the Medical Devices Agency (MDA). In April 2013, it merged with the National Institute for Biological Standards and Control (NIBSC) and was rebranded, with the MHRA identity being used solely for the regulatory centre within the group. The agency employs more than 1,200 people in London, York and South Mimms, Hertfordshire. Structure The MHRA is divided into three main centres: * MHRA Regulatory – the regulator for the pharmaceutical and medical devices industries * Clinical Practice Research Datalink – licences anonymised health care data to pharmaceutical companies, academics and other regulators for research * National Institute for Biolog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WHOART
The WHO Adverse Reactions Terminology (WHOART) is a dictionary meant to serve as a basis for rational coding of adverse reaction terms. The system is maintained by the Uppsala Monitoring Centre (UMC), the World Health Organization Collaborating Centre for International Drug Monitoring. The system is no longer actively maintained. Structure * 32 System-organ classesbody organ groups * 180 High level terms for grouping Preferred terms * 2085 Preferred terms principal terms for describing adverse reactions * 3445 Included terms synonyms to Preferred terms See also *Pharmacovigilance *COSTART *MedDRA *Adverse event An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can ther ... References WHO Adverse Reactions Terminology Medical classification Pharmacological classification systems {{treatm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SNOMED CT
SNOMED CT or SNOMED Clinical Terms is a systematically organized computer-processable collection of medical terms providing codes, terms, synonyms and definitions used in clinical documentation and reporting. SNOMED CT is considered to be the most comprehensive, multilingual clinical healthcare terminology in the world. The primary purpose of SNOMED CT is to encode the meanings that are used in health information and to support the effective clinical recording of data with the aim of improving patient care. SNOMED CT provides the core general terminology for electronic health records. SNOMED CT comprehensive coverage includes: clinical findings, symptoms, diagnoses, procedures, body structures, organisms and other etiologies, substances, pharmaceuticals, devices and specimens. SNOMED CT is maintained and distributed by SNOMED International, an international non-profit standards development organization, located in London, UK. SNOMED International is the trading name of the Intern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Systematized Nomenclature Of Medicine
The Systematized Nomenclature of Medicine (SNOMED) is a systematic, computer-processable collection of medical terms, in human and veterinary medicine, to provide codes, terms, synonyms and definitions which cover anatomy, diseases, findings, procedures, microorganisms, substances, etc. It allows a consistent way to index, store, retrieve, and aggregate medical data across specialties and sites of care. Although now international, SNOMED was started in the U.S. by the College of American Pathologists (CAP) in 1973 and revised into the 1990s. In 2002 CAP's SNOMED Reference Terminology (SNOMED RT) was merged with, and expanded by, the National Health Service's Clinical Terms Version 3 (previously known as the Read codes) to produce SNOMED CT. Versions of SNOMED released prior to 2001 were based on a multiaxial, hierarchical classification system. As in any such system, a disease may be located in a body organ (anatomy), which results in a code in a topography axis and may lead to m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COSTART
The Coding Symbols for a Thesaurus of Adverse Reaction Terms (COSTART) was developed by the United States Food and Drug Administration (FDA) for the coding, filing and retrieving of post-marketing adverse reaction reports. COSTART provides a method to deal with the variation in vocabulary used by those who submit adverse event reports to the FDA. Use of this dictionary allowed for standardization of adverse reaction reporting towards the FDA in a consistent way. COSTART was last updated in 1999. It has been replaced by the Medical Dictionary for Regulatory Activities, MedDRA. See also * Pharmacovigilance * WHOART * Adverse event An adverse event (AE) is any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. An adverse event can ther ... References External links COSTART lookup {{treatment-stub Pharmacological classification systems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Commerce
Commerce is the large-scale organized system of activities, functions, procedures and institutions directly and indirectly related to the exchange (buying and selling) of goods and services among two or more parties within local, regional, national or international economies. More specifically, commerce is not business, but rather the part of business which facilitates the movement and distribution of finished or unfinished but valuable goods and services from the producers to the end consumers on a large scale, as opposed to the sourcing of raw materials and manufacturing of those goods. Commerce is subtly different from trade as well, which is the final transaction, exchange or transfer of finished goods and services between a seller and an end consumer. Commerce not only includes trade as defined above, but also a series of transactions that happen between the producer and the seller with the help of the auxiliary services and means which facilitate such trade. These auxiliary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)
