|
Malaprade Reaction
In organic chemistry, the Malaprade reaction or Malaprade oxidation is a glycol cleavage reaction in which a vicinal diol is oxidized by periodic acid or a periodate salt to give the corresponding carbonyl functional groups. The reaction was first reported by in 1928 and also works with β-aminoalcohols. File:Malaprade.svg, Malaprade reaction File:Glykolspaltung RMa cis trans Malaprade.svg, Malaprade reaction mechanism References See also * Criegee oxidation The Criegee oxidation is a glycol cleavage reaction in which vicinal diols are oxidized to form ketones and aldehydes using lead tetraacetate. It is analogous to the Malaprade reaction, but uses a milder oxidant. This oxidation was discovere ... {{organic-chem-stub Organic oxidation reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycol Cleavage
Glycol cleavage is a specific type of organic chemistry oxidation. The carbon–carbon bond in a vicinal diol (glycol) is cleaved and instead the two oxygen atoms become double-bonded to their respective carbon atoms. Depending on the substitution pattern in the diol, these carbonyls can be either ketones or aldehydes. Glycol cleavage is an important reaction in the laboratory because it is useful for determining the structures of sugars. After cleavage takes place the ketone and aldehyde fragments can be inspected and the location of the former hydroxyl groups ascertained. Reagents Periodic acid (HIO4), (diacetoxyiodo)benzene (PhI(OAc)2) and lead tetraacetate (Pb(OAc)4) are the most common reagents used for glycol cleavage, processes called the Malaprade reaction and Criegee oxidation, respectively. These reactions are most efficient when a cyclic intermediate can form, with the iodine or lead atom linking both oxygen atoms. The ring then fragments, with breakage of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vicinal (chemistry)
In chemistry the descriptor vicinal (from Latin ''vicinus'' = neighbor), abbreviated ''vic'', describes any two functional groups bonded to two adjacent carbon atoms (i.e., in a 1,2-relationship). Relation of atoms in a molecule For example, the molecule 2,3-dibromobutane carries two vicinal bromine atoms and 1,3-dibromobutane does not. Mostly, the use of the term vicinal is restricted to two ''identical'' functional groups. Likewise in a ''gem-''dibromide the prefix ''gem'', an abbreviation of geminal, signals that both bromine atoms are bonded to the ''same'' atom (i.e., in a 1,1-relationship). For example, 1,1-dibromobutane is geminal. While comparatively less common, the term hominal has been suggested as a descriptor for groups in a 1,3-relationship. Like other such descriptors as syn, anti, exo or endo, the description ''vicinal'' helps explain how different parts of a molecule are related to each other either structurally or spatially. The vicinal adjective is sometim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diol
A diol is a chemical compound containing two hydroxyl groups ( groups). An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified. The most common industrial diol is ethylene glycol. Examples of diols in which the hydroxyl functional groups are more widely separated include 1,4-butanediol and propylene-1,3-diol, or beta propylene glycol, . Synthesis of classes of diols Geminal diols A geminal diol has two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. The hydration is usually unfavorable, but a notable exception is formaldehyde which, in water, exists in equilibrium with methanediol H2C(OH)2. Another example is (F3C)2C(OH)2, the hydrated form of hexafluoroacetone. Many gem-diols undergo further condensation to give dimeric and oligomeric derivatives. This reaction applies to glyoxal and related aldehydes. Vicinal diols In a vicinal diol, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Oxidation
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodic Acid
Periodic acid ( ) is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. Like all periodates it can exist in two forms: orthoperiodic acid, with the chemical formula , and metaperiodic acid, which has the formula . Periodic acid was discovered by Heinrich Gustav Magnus and C. F. Ammermüller in 1833. Synthesis Modern industrial scale production involves the oxidation of a solution of sodium iodate under alkaline conditions, either electrochemically on a anode, or by treatment with chlorine: : (counter ions omitted for clarity) ''E''° = -1.6 V : Orthoperiodic acid can be dehydrated to give metaperiodic acid by heating to 100 °C under reduced pressure. : Further heating to around 150 °C gives iodine pentoxide () rather than the expected anhydride ''diiodine heptoxide'' (). Metaperiodic acid can also be prepared from various orthoperiodates by treatment with dilute nitric acid. Properties Orthoperiodic acid has a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periodate
Periodate is an anion composed of iodine and oxygen. It is one of a number of oxyanions of iodine and is the highest in the series, with iodine existing in oxidation state +7. Unlike other perhalogenates, such as perchlorate, it can exist in two forms: metaperiodate and orthoperiodate . In this regard it is comparable to the tellurate ion from the adjacent group. It can combine with a number of counter ions to form periodates, which may also be regarded as the salts of periodic acid. Periodates were discovered by Heinrich Gustav Magnus and C. F. Ammermüller; who first synthesised periodic acid in 1833. Synthesis Classically, periodate was most commonly produced in the form of sodium hydrogen periodate (). This is commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide. Or, similarly, from iodides by oxidation with bromine and sodium hydroxide: :\overset + Cl2 + 4 NaOH -> Na3H2IO6 + 2NaCl + H2O :NaI + 4 Br2 + 10 NaOH -> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminoalcohol
In organic chemistry, alkanolamines are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification. Methanolamine.svg, methanolamine, an intermediate in the reaction of ammonia with formaldehyde Ethanolamine.png, Ethanolamine 2-amino-2-methyl-1-propanol.svg, 2-amino-2-methyl-1-propanol is a precursor to oxazolines valinol.svg, valinol is derived from the amino acid valine Sphingosine structure.svg, Sphingosine is a component of some cell membrane. 1-Aminoalcohols 1-Aminoalcohols are better known as hemiaminals. Methanolamine is the simplest member. 2-Aminoalcohols Key members: ethanolamine, dimethylethanolamine, N-Methylethanolamine, ''N''-methylethanolamine, Aminomethyl propanol Two popular drugs, often called alkanolamine beta blockers, are members of this structural class: propranolol, pindolol. Isoetarine is yet another medicinall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
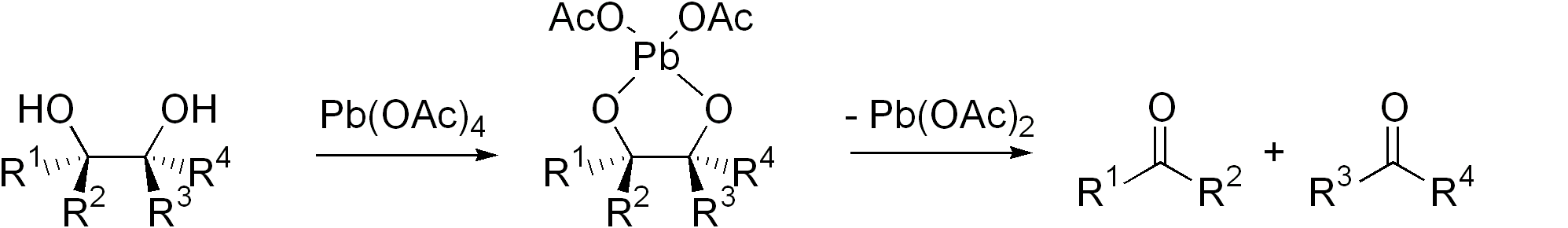
Criegee Oxidation
The Criegee oxidation is a glycol cleavage reaction in which vicinal diols are oxidized to form ketones and aldehydes using lead tetraacetate. It is analogous to the Malaprade reaction, but uses a milder oxidant. This oxidation was discovered by Rudolf Criegee and coworkers and first reported in 1931 using ethylene glycol as the substrate. The rate of the reaction is highly dependent on the relative geometric position of the two hydroxyl groups, so much so that diols that are ''cis'' on certain rings can be reacted selectively as opposed to those that are ''trans'' on them. It was heavily stressed by Criegee that the reaction must be run in anhydrous solvents, as any water present would hydrolyze the lead tetraacetate; however, subsequent publications have reported that if the rate of oxidation is faster than the rate of hydrolysis, the cleavage can be run in wet solvents or even aqueous solutions. For example, glucose, glycerol, mannitol, and xylose can all undergo a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Oxidation Reactions
Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product of decay, or is composed of organic compounds * Organic compound, a compound that contains carbon ** Organic chemistry, chemistry involving organic compounds Farming, certification and products * Organic farming, agriculture conducted according to certain standards, especially the use of stated methods of fertilization and pest control * Organic certification, accreditation process for producers of organically-farmed products * Organic horticulture, the science and art of growing fruits, vegetables, flowers, or ornamental plants by following the essential principles of organic agriculture * Organic products, "organics": ** Organic food, food produced from organic farming methods and often certified organic according to organic farming stan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |