|
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from ''magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Periclase
Periclase is a magnesium mineral that occurs naturally in Contact metamorphism, contact metamorphic rocks and is a major component of most basic refractory bricks. It is a Cubic crystal system, cubic form of magnesium oxide (magnesium, Mgoxygen, O). In nature it usually forms a solid solution with wüstite (FeO) and is then referred to as ferropericlase or magnesiowüstite. It was first described in 1840 and named from the Greek περικλάω (to break around) in allusion to its cleavage. The Type locality (geology), type locality is Monte Somma, Somma-Vesuvius Complex, Naples Province, Campania, Italy. The old term for the mineral is ''magnesia''. Stones from the Magnesia on the Maeander, Magnesia region in ancient Anatolia contained both magnesium oxide and hydrated magnesium carbonate as well as iron oxides (such as magnetite). Thus these stones, called ''Stones from Magnesia'' in antiquity, with their unusual magnetic properties were the reason the terms ''magnet'' and ''m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygroscopy
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Room Temperature
Colloquially, "room temperature" is a range of air temperatures that most people prefer for indoor settings. It feels comfortable to a person when they are wearing typical indoor clothing. Human comfort can extend beyond this range depending on humidity, air circulation and other factors. Food or beverages may be served at ''room temperature'', meaning neither heated nor cooled. In certain fields, like science and engineering, and within a particular context, ''room temperature'' can mean different agreed-upon ranges. In contrast, ''ambient temperature'' is the actual temperature, as measured by a thermometer, of the air (or other medium and surroundings) in any particular place. The ambient temperature (e.g. an unheated room in winter) may be very different from an ideal ''room temperature''. Comfort temperatures ''The American Heritage Dictionary of the English Language'' identifies room temperature as around , while the ''Oxford English Dictionary'' states that it is "conv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suboxide
Suboxides are a class of oxides wherein the electropositive element is in excess relative to the “normal” oxides. When the electropositive element is a metal, the compounds are sometimes referred to as “metal-rich”. Thus the normal oxide of caesium is Cs2O, which is described as a Cs+ salt of O2−. A suboxide of caesium is Cs11O3, where the charge on Cs is clearly less than 1+, but the oxide is still described as O2−. Suboxides typically feature extensive bonding between the electropositive element, often leading to clusters. Examples of suboxides other than alkali metal derivatives: * Boron suboxide, BO; * Carbon suboxide, C3O2; * Boron suboxide, B6O; * Phosphorus suboxide, PO Metal-containing suboxides Suboxides are intermediates along the pathway that forms the normal oxide. Suboxides are sometimes visible when certain metals are exposed to small amounts of O2: :22 Cs + 3 O2 → 2 Cs11O3 :4 Cs11O3 + 5 O2 → 22 Cs2O Several suboxides of caesium and rub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Peroxide
Magnesium peroxide (MgO2) is an odorless fine powder peroxide with a white to off-white color. It is similar to calcium peroxide because magnesium peroxide also releases oxygen by breaking down at a controlled rate with water. Commercially, magnesium peroxide often exists as a compound of magnesium peroxide and magnesium hydroxide. Structure O2, similarly to N2, has the ability to bind either side-on or end-on. The structure of MgO2 has been calculated as a triangular shape with the O2 molecule binding side-on to the magnesium. This arrangement is a result of the Mg+ donating charge to the oxygen and creating a Mg2+O22−. The bond between to O2 and the magnesium atom has an approximate dissociation energy of 90 kJ mol−1. In the solid state, MgO2 has a cubic pyrite-type crystal structure with 6-coordinate Mg2+ ions and O22− peroxide-groups, according to experimental data and evolutionary crystal structure prediction, the latter predicting a phase transition at the pressure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese was first isolated in 1774. It is familiar in the laboratory in the form of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesia Negra
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy uses, particularly in stainless steels. It improves strength, workability, and resistance to wear. Manganese oxide is used as an oxidising agent; as a rubber additive; and in glass making, fertilisers, and ceramics. Manganese sulfate can be used as a fungicide. Manganese is also an essential human dietary element, important in macronutrient metabolism, bone formation, and free radical defense systems. It is a critical component in dozens of proteins and enzymes. It is found mostly in the bones, but also the liver, kidneys, and brain. In the human brain, the manganese is bound to manganese metalloproteins, most notably glutamine synthetase in astrocytes. Manganese was first isolated in 1774. It is familiar in the laboratory in the form of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
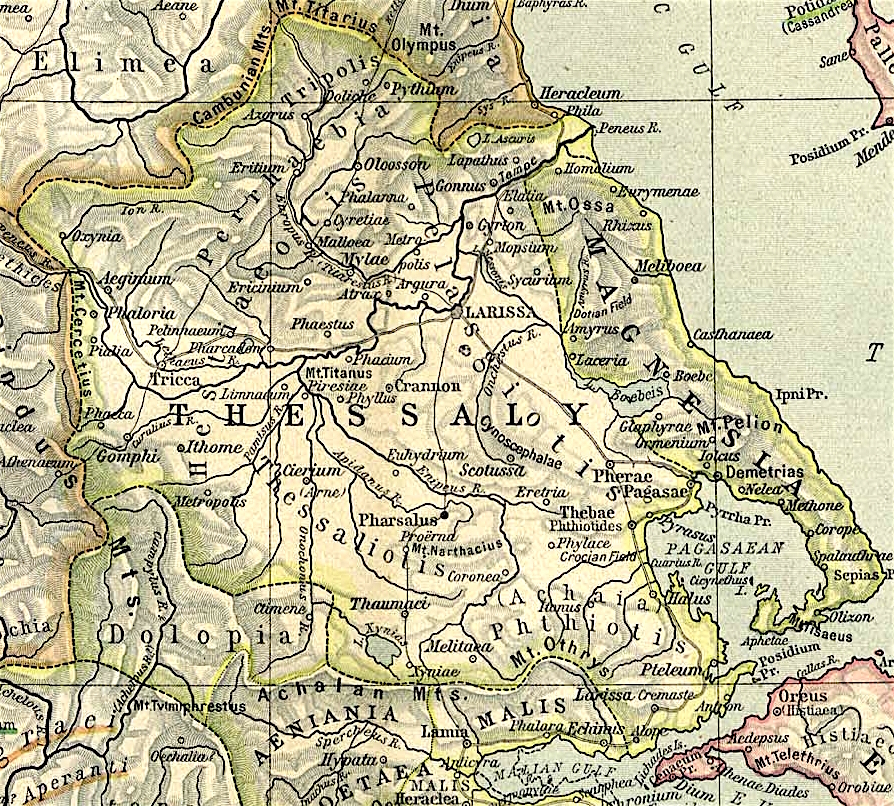
Ancient Magnesia
Anciently, Magnesia ( grc, Μαγνησία) was a region of Ancient Greece, eventually absorbed by ancient Thessaly. Originally inhabited by the Magnetes (Μάγνητες), Magnesia was the long and narrow slip of country between Mounts Ossa and Pelion on the west and the sea on the east, and extending from the mouth of the Peneius on the north to the Pagasaean Gulf on the south. The Magnetes were members of the Amphictyonic League, and were settled in this district in the Homeric times, and mentioned in the ''Iliad''. The Thessalian Magnetes are said to have founded the Asiatic cities of Magnesia ad Sipylum and Magnesia on the Maeander.Aristot. ''ap. Athen.'' 4.173; Conon 29; The towns of Magnesia were: Aesonis, Aphetae, Boebe, Casthanaea, Cercinium, Coracae, Demetrias, Eurymenae, Glaphyrae, Homole or Homolium, Iolcus, Magnesia, Meliboea, Methone, Mylae, Nelia, Olizon, Pagasae, Rhizus, Spalaethra, and Thaumacia Thaumacia or Thaumakie ( grc, Θαυμακία or Θαυ� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Bond
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with sharply different electronegativities, and is the primary interaction occurring in ionic compounds. It is one of the main types of bonding along with covalent bonding and metallic bonding. Ions are atoms (or groups of atoms) with an electrostatic charge. Atoms that gain electrons make negatively charged ions (called anions). Atoms that lose electrons make positively charged ions (called cations). This transfer of electrons is known as electrovalence in contrast to covalence. In the simplest case, the cation is a metal atom and the anion is a nonmetal atom, but these ions can be of a more complex nature, e.g. molecular ions like or . In simpler words, an ionic bond results from the transfer of electrons from a metal to a non-metal in order to obtain a full valence shell for both atoms. It is important to recognize that ''clean'' ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Formula
In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, would simply be SO, as is the empirical formula of disulfur dioxide, S2O2. Thus, sulfur monoxide and disulfur dioxide, both compounds of sulfur and oxygen, have the same empirical formula. However, their molecular formulas, which express the number of atoms in each molecule of a chemical compound, are not the same. An empirical formula makes no mention of the arrangement or number of atoms. It is standard for many ionic compounds, like calcium chloride (CaCl2), and for macromolecules, such as silicon dioxide (SiO2). The molecular formula, on the other hand, shows the number of each type of atom in a molecule. The structural formula shows the arrangement of the molecule. It is also possible for different types of compounds to have equal empirical formulas. Sampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |