|
MAD1
Mad1 is a non-essential protein which in yeast has a function in the spindle assembly checkpoint (SAC). This checkpoint monitors chromosome attachment to spindle microtubules and prevents cells from starting anaphase until the spindle is built up. The name Mad refers to the observation that mutant cells are mitotic arrest deficient (MAD) during microtubule depolymerization. Mad1 recruits the anaphase inhibitor Mad2 to unattached kinetochores and is essential for Mad2-Cdc20 complex formation ''in vivo'' but not ''in vitro''. ''In vivo'', Mad1 acts as a competitive inhibitor of the Mad2-Cdc20 complex. Mad1 is phosphorylated by Mps1 which then leads together with other activities to the formation of the mitotic checkpoint complex (MCC). Thereby it inhibits the activity of the anaphase-promoting complex/cyclosome (APC/C). Homologues of Mad1 are conserved in eukaryotes from yeast to mammals. Introduction In the early 90s, yeast genes were identified which mutations resulted in a de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAD1 Function In SAC
Mad1 is a non-essential protein which in yeast has a function in the spindle assembly checkpoint (SAC). This checkpoint monitors chromosome attachment to spindle microtubules and prevents cells from starting anaphase until the spindle is built up. The name Mad refers to the observation that mutant cells are mitotic arrest deficient (MAD) during microtubule depolymerization. Mad1 recruits the anaphase inhibitor Mad2 to unattached kinetochores and is essential for Mad2-Cdc20 complex formation ''in vivo'' but not ''in vitro''. ''In vivo'', Mad1 acts as a competitive inhibitor of the Mad2-Cdc20 complex. Mad1 is phosphorylated by Mps1 which then leads together with other activities to the formation of the mitotic checkpoint complex (MCC). Thereby it inhibits the activity of the anaphase-promoting complex/cyclosome (APC/C). Homologues of Mad1 are conserved in eukaryotes from yeast to mammals. Introduction In the early 90s, yeast genes were identified which mutations resulted in a de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mad2
Mad2 (mitotic arrest deficient 2) is an essential spindle checkpoint protein. The spindle checkpoint system is a regulatory system that restrains progression through the metaphase-to-anaphase transition. The Mad2 gene was first identified in the yeast ''S. cerevisiae'' in a screen for genes which when mutated would confer sensitivity to microtubule poisons. The human orthologues of Mad2 (MAD2L1 and MAD2L2) were first cloned in a search for human cDNAs that would rescue the microtubule poison-sensitivity of a yeast strain in which a kinetochore binding protein was missing. The protein was shown to be present at unattached kinetochores and antibody inhibition studies demonstrated it was essential to execute a block in the metaphase-to-anaphase transition in response to the microtubule poison nocodazole. Subsequent cloning of the ''Xenopus laevis'' orthologue, facilitated by the sharing of the human sequence, allowed for the characterization of the mitotic checkpoint in egg extracts. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAD2
Mad2 (mitotic arrest deficient 2) is an essential spindle checkpoint protein. The spindle checkpoint system is a regulatory system that restrains progression through the metaphase-to-anaphase transition. The Mad2 gene was first identified in the yeast ''S. cerevisiae'' in a screen for genes which when mutated would confer sensitivity to microtubule poisons. The human orthologues of Mad2 (MAD2L1 and MAD2L2) were first cloned in a search for human cDNAs that would rescue the microtubule poison-sensitivity of a yeast strain in which a kinetochore binding protein was missing. The protein was shown to be present at unattached kinetochores and antibody inhibition studies demonstrated it was essential to execute a block in the metaphase-to-anaphase transition in response to the microtubule poison nocodazole. Subsequent cloning of the ''Xenopus laevis'' orthologue, facilitated by the sharing of the human sequence, allowed for the characterization of the mitotic checkpoint in egg extracts. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetochores
A kinetochore (, ) is a disc-shaped protein structure associated with duplicated chromatids in eukaryotic cells where the spindle fibers attach during cell division to pull sister chromatids apart. The kinetochore assembles on the centromere and links the chromosome to microtubule polymers from the mitotic spindle during mitosis and meiosis. The term kinetochore was first used in a footnote in a 1934 Cytology book by Lester W. Sharp and commonly accepted in 1936. Sharp's footnote reads: "The convenient term ''kinetochore'' (= movement place) has been suggested to the author by J. A. Moore", likely referring to John Alexander Moore who had joined Columbia University as a freshman in 1932. Monocentric organisms, including vertebrates, fungi, and most plants, have a single centromeric region on each chromosome which assembles a single, localized kinetochore. Holocentric organisms, such as nematodes and some plants, assemble a kinetochore along the entire length of a chromosome. Ki ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
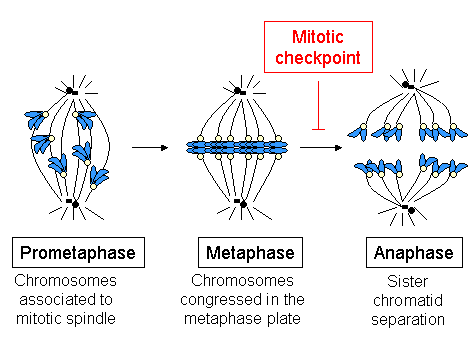
Spindle Assembly Checkpoint
The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles (bipolar orientation). Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together. Overview and importance The beginning of metaphase is characterized by the connection of the microtubules to the kinetochores of the chromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spindle Checkpoint
The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles (bipolar orientation). Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together. Overview and importance The beginning of metaphase is characterized by the connection of the microtubules to the kinetochores of the chrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spindle Assembly Checkpoint
The spindle checkpoint, also known as the metaphase-to-anaphase transition, the spindle assembly checkpoint (SAC), the metaphase checkpoint, or the mitotic checkpoint, is a cell cycle checkpoint during mitosis or meiosis that prevents the separation of the duplicated chromosomes (anaphase) until each chromosome is properly attached to the spindle. To achieve proper segregation, the two kinetochores on the sister chromatids must be attached to opposite spindle poles (bipolar orientation). Only this pattern of attachment will ensure that each daughter cell receives one copy of the chromosome. The defining biochemical feature of this checkpoint is the stimulation of the anaphase-promoting complex by M-phase cyclin-CDK complexes, which in turn causes the proteolytic destruction of cyclins and proteins that hold the sister chromatids together. Overview and importance The beginning of metaphase is characterized by the connection of the microtubules to the kinetochores of the chromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BUB1
Mitotic checkpoint serine/threonine-protein kinase BUB1 also known as BUB1 (budding uninhibited by benzimidazoles 1) is an enzyme that in humans is encoded by the ''BUB1'' gene. Bub1 is a serine/threonine protein kinase first identified in genetic screens of ''Saccharomyces cerevisiae''. The protein is bound to kinetochores and plays a key role in the establishment of the mitotic spindle checkpoint and chromosome congression. The mitotic checkpoint kinase is evolutionarily conserved in organisms as diverse as ''Saccharomyces cerevisiae'' and humans. Loss-of-function mutations or absence of Bub1 has been reported to result in aneuploidy, chromosomal instability ( CIN) and premature senescence. Structure Bub1p comprises a conserved N-terminal region, a central non-conserved region and a C-terminal serine/threonine kinase domain. The N-terminal region mediates binding of Hs-BUB1 to the mitotic kinetochore protein blinkin (a protein also commonly referred to as AF15q14). The latte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cdc20
The cell division cycle protein 20 homolog is an essential regulator of cell division that is encoded by the ''CDC20'' gene in humans. To the best of current knowledge its most important function is to activate the anaphase promoting complex (APC/C), a large 11-13 subunit complex that initiates chromatid separation and entrance into anaphase. The APC/CCdc20 protein complex has two main downstream targets. Firstly, it targets securin for destruction, enabling the eventual destruction of cohesin and thus sister chromatid separation. It also targets S and M-phase (S/M) cyclins for destruction, which inactivates S/M cyclin-dependent kinases (Cdks) and allows the cell to exit from mitosis. A closely related protein, Cdc20homologue-1 (Cdh1) plays a complementary role in the cell cycle. CDC20 appears to act as a regulatory protein interacting with many other proteins at multiple points in the cell cycle. It is required for two microtubule-dependent processes: nuclear movement prior to an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperphosphorylation
Hyperphosphorylation occurs when a biochemical with multiple phosphorylation sites is fully saturated. Hyperphosphorylation is one of the signaling mechanisms used by the cell to regulate mitosis. When these mechanisms fail, developmental problems or cancer are a likely outcome. The mechanism appears to be largely conserved throughout eukaryote species. The dynamics of mitosis are similar to a state machine. In a healthy cell, checkpoints between phases permit a new phase to begin only when the previous phase is complete and successful. At these checkpoints, gatekeeper molecules block or allow events, depending on their level of phosphorylation. Kinases are responsible for adding phosphate groups and phosphatases for removing them. Cyclins are molecules that manage the timing of cell cycle events. Cyclin dependent kinases pair up with cyclins to become operational. Cyclins are named because they are created or destroyed at predetermined points within the cell cycle. Kinase inhi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anaphase-promoting Complex
Anaphase-promoting complex (also called the cyclosome or APC/C) is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin (Apc2) and RING (Apc11) subunit much like SCF. Other parts of the APC/C have unknown functions but are highly conserved. It was the discovery of the APC/C (and SCF) and their key role in eukaryotic cell-cycle regulation that established the importance of ubiquitin-mediated proteolysis in cell biology. Once perceived as a system exclusively involved in removing damaged protein from the cell, ubiquitination and subsequent protein degradation by the proteasome is now perceived as a universal regulatory mechanism for signal transduction whose importance approaches that of protein phosphorylation. In 2014, the APC/C was mapped in 3D at a resolution of less than a nanometre, which also uncovered its secondary structure. This finding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinases
In biochemistry, a kinase () is an enzyme that catalysis, catalyzes the transfer of phosphate groups from High-energy phosphate, high-energy, phosphate-donating molecules to specific Substrate (biochemistry), substrates. This process is known as phosphorylation, where the high-energy adenosine triphosphate, ATP molecule donates a phosphate group to the substrate (biology), substrate molecule. This transesterification produces a phosphorylated substrate and Adenosine diphosphate, ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and adenosine diphosphate, ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |