|
MLCK
Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II. General structural features While there are numerous differing domains depending on the cell type, there are several characteristic domains common amongst all MYLK isoforms. MYLK’s contain a catalytic core domain with an ATP binding domain. On either sides of the catalytic core sit calcium ion/calmodulin binding sites. Binding of calcium ion to this domain increases the affinity of MYLK binding to myosin light chain. This myosin binding domain is located at the C-Terminus end of the kinase. On the other side of the kinase at the N-Terminus end, sits the actin-binding domain, which allows MYLK to form interactions with actin filaments, keeping it in place. Isoforms Four different MYLK isoforms exist: * MYLK1 – smooth muscle * MYLK2 – skeletal * MYLK3 – cardiac * MYLK4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYLK2
Myosin light chain kinase 2 also known as MYLK2 is an enzyme which in humans is encoded by the ''MYLK2'' gene. Function This gene encodes a myosin light chain kinase, a calcium / calmodulin dependent enzyme, that is exclusively expressed in adult skeletal muscle. The MYLK2 gene expresses skMLCK more prevalently in fast twitch muscle fibers as compared to slow twitch muscle fibers. Calmodulin is composed of two terminal domains (N,C) each containing two E-F hand motifs that bind to Ca2+. Upon saturation of Ca2+, Calmodulin undergoes a conformation change allowing it to bind with a target protein such as skMLCK. An image depicting a similar complex (sdCen/skMLCK2) is shown under myosin light chain kinase. This binding to skMLCK increases the affinity of Ca2+ and ultimately leads to a sustained muscle action. Clinical significance Mutations in the ''MYLK2'' gene have been linked to midventricular hypertrophic cardiomyopathy Hypertrophic cardiomyopathy (HCM, or HOCM ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin Light Chain
A myosin light chain is a light chain (small polypeptide subunit) of myosin. Myosin light chains were discovered by Chinese biochemist Cao Tianqin (Tien-chin Tsao) when he was a graduate student at the University of Cambridge in England. Structure and function Myosin light chain classes Structurally, myosin light chains belong to the EF-hand family, a large family of Ca2+- binding proteins. MLCs contain two Ca2+ - binding EF-hand motifs. MLCs isoforms modulate the Ca2+of force transduction and cross-bridge kinetics. Myosin light chains (MLCs) can be broadly classified into two groups: * Essential or alkali MLC (MLC1 or ELC), * Regulatory MLC (MLC2 or RLC). Essential and regulatory MLCs have molecular masses of 22 and 19 kDa, respectively. Structurally, MLC2 contains a serine residue that is lacking in MLC1. The presence of this amino acids allows the regulation of the conformational changes (from compacted to an elongated form) by a Ca2+-mediated phosphorylation mechanism ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Centrin
Centrins, also known as caltractins, are a family of calcium-binding phosphoproteins found in the centrosome of eukaryotes. Centrins are present in the centrioles and pericentriolar lattice. Human centrin genes are CETN1, CETN2 and CETN3. History Centrin was first isolated and characterized from the flagellar roots of the green alga ''Tetraselmis striata'' in 1984. Function Centrins are required for duplication of centrioles. They may also play a role in severing of microtubules by causing calcium-mediated contraction. The majority of centrin in the cell is non-centrosomal whose function is not yet clear. Structure Centrin belongs to the EF-hand superfamily of calcium-binding proteins and has four calcium-binding EF-hands. It has a molecular weight of 20 kDa. See also * Centriole * Centrosome In cell biology, the centrosome (Latin centrum 'center' + Greek sōma 'body') (archaically cytocentre) is an organelle that serves as the main microtubule organizing center (MTOC) of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYLK3
Myosin light chain kinase 3 also known as MYLK3, is an enzyme which in humans is encoded by the ''MYLK3'' gene. Function Phosphorylation of cardiac myosin heavy chains (see MYH7B) and light chains (see MYL2) by a kinase, such as MYLK3, potentiates the force and rate of cross-bridge recruitment in cardiac myocytes. See also * Myosin light-chain kinase Myosin light-chain kinase also known as MYLK or MLCK is a serine/threonine-specific protein kinase that phosphorylates a specific myosin light chain, namely, the regulatory light chain of myosin II. General structural features While there ar ... References Further reading * * * * * * * * * * EC 2.7.11 {{gene-16-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYLK
Myosin light chain kinase, smooth muscle also known as kinase-related protein (KRP) or telokin is an enzyme that in humans is encoded by the ''MYLK'' gene. Function This gene, a muscle member of the immunoglobulin superfamily, encodes a myosin light-chain kinase, which is a calcium-/calmodulin-dependent enzyme. This kinase phosphorylates myosin regulatory light chains to facilitate myosin interaction with actin filaments to produce contractile activity. This gene encodes both smooth muscle and nonmuscle isoforms. In addition, using a separate promoter in an intron in the 3' region, it encodes telokin, a small protein identical in sequence to the C-terminus of myosin light chain kinase, that is independently expressed in smooth muscle and functions to stabilize unphosphorylated myosin filaments. A pseudogene is located on the p arm of chromosome 3. Four transcript variants that produce four isoforms of the calcium/calmodulin dependent enzyme have been identified as well as two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proto-oncogene Tyrosine-protein Kinase Src
Proto-oncogene tyrosine-protein kinase Src, also known as proto-oncogene c-Src, or simply c-Src (cellular Src; pronounced "sarc", as it is short for sarcoma), is a non-receptor tyrosine kinase protein that in humans is encoded by the ''SRC'' gene. It belongs to a family of Src family kinases and is similar to the v-Src (viral Src) gene of Rous sarcoma virus. It includes an SH2 domain, an SH3 domain and a tyrosine kinase domain. Two transcript variants encoding the same protein have been found for this gene. c-Src phosphorylates specific tyrosine residues in other tyrosine kinases. It plays a role in the regulation of embryonic development and cell growth. An elevated level of activity of c-Src is suggested to be linked to cancer progression by promoting other signals. Mutations in c-Src could be involved in the malignant progression of colon cancer. c-Src should not be confused with CSK (C-terminal Src kinase), an enzyme that phosphorylates c-Src at its C-terminus and provides n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYLK4
Myosin light chain kinase 4 also known as MYLK4 is an enzyme which in humans is encoded by the ''MYLK2'' gene. MYLK4 is a member of the myosin light-chain kinase family of serine/threonine-specific protein kinases that phosphorylate the regulatory light chain of myosin II. This protein acts as an enzyme that catalyzes the following reaction: ATP + a protein -> ADP + a phosphoprotein. MYLK4 is also involved in protein amino acid phosphorylation meaning that it adds a phosphate group onto the molecule. Not very much is known about the specific functional characteristics of MLYK4, but it has recently been found that the gene may possibly have a role in having at least one driver mutation for cancer. MYLK4 may also be involved in transferase activity, ATP binding, protein serine/threonine kinase activity, and also nucleotide binding. Other names for MYLK4 are CaMLCK like; EG238564; MYLK4; Mylk4; Myosin light chain kinase family, member 4; SgK085; SGK085; Sgk085; Sugen kinase 85; Unch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine/threonine-specific Protein Kinase
A serine/threonine protein kinase () is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK). In enzymology, the term ''serine/threonine protein kinase'' describes a class of enzymes in the family of transferases, that transfer phosphates to the oxygen atom of a serine or threonine side chain in proteins. This process is called phosphorylation. Protein phosphorylation in particular plays a significant role in a wide range of cellular processes and is a very important posttranslational modification. The chemical reaction performed by these enzymes can be written as :ATP + a protein \rightleftharpoons ADP + a phosphoprotein Thus, the two substrates of this enzyme are ATP and a protein, whereas its two products are ADP and phosphoprotein. The systematic name of this enzyme class is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin Filament
Microfilaments, also called actin filaments, are protein filaments in the cytoplasm of eukaryotic cells that form part of the cytoskeleton. They are primarily composed of polymers of actin, but are modified by and interact with numerous other proteins in the cell. Microfilaments are usually about 7 nm in diameter and made up of two strands of actin. Microfilament functions include cytokinesis, amoeboid movement, cell motility, changes in cell shape, endocytosis and exocytosis, cell contractility, and mechanical stability. Microfilaments are flexible and relatively strong, resisting buckling by multi-piconewton compressive forces and filament fracture by nanonewton tensile forces. In inducing cell motility, one end of the actin filament elongates while the other end contracts, presumably by myosin II molecular motors. Additionally, they function as part of actomyosin-driven contractile molecular motors, wherein the thin filaments serve as tensile platforms for myosin's ATP-depend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extra Cellular Matrix
In biology, the extracellular matrix (ECM), also called intercellular matrix, is a three-dimensional network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM. The animal extracellular matrix includes the interstitial matrix and the basement membrane. Interstitial matrix is present between various animal cells (i.e., in the intercellular spaces). Gels of polysaccharides and fibrous proteins fill the interstitial space and act as a compression buffer against the stress placed on the ECM. Basement membranes are sheet-like depositions of ECM on which various epithelial cells rest. Each type of connectiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adherens Junction
Adherens junctions (or zonula adherens, intermediate junction, or "belt desmosome") are protein complexes that occur at cell–cell junctions, cell–matrix junctions in epithelial and endothelial tissues, usually more basal than tight junctions. An adherens junction is defined as a cell junction whose cytoplasmic face is linked to the actin cytoskeleton. They can appear as bands encircling the cell (zonula adherens) or as spots of attachment to the extracellular matrix (focal adhesion). Adherens junctions uniquely disassemble in uterine epithelial cells to allow the blastocyst to penetrate between epithelial cells. A similar cell junction in non-epithelial, non-endothelial cells is the fascia adherens. It is structurally the same, but appears in ribbonlike patterns that do not completely encircle the cells. One example is in cardiomyocytes. Proteins Adherens junctions are composed of the following proteins: * cadherins. The cadherins are a family of transmembrane proteins tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
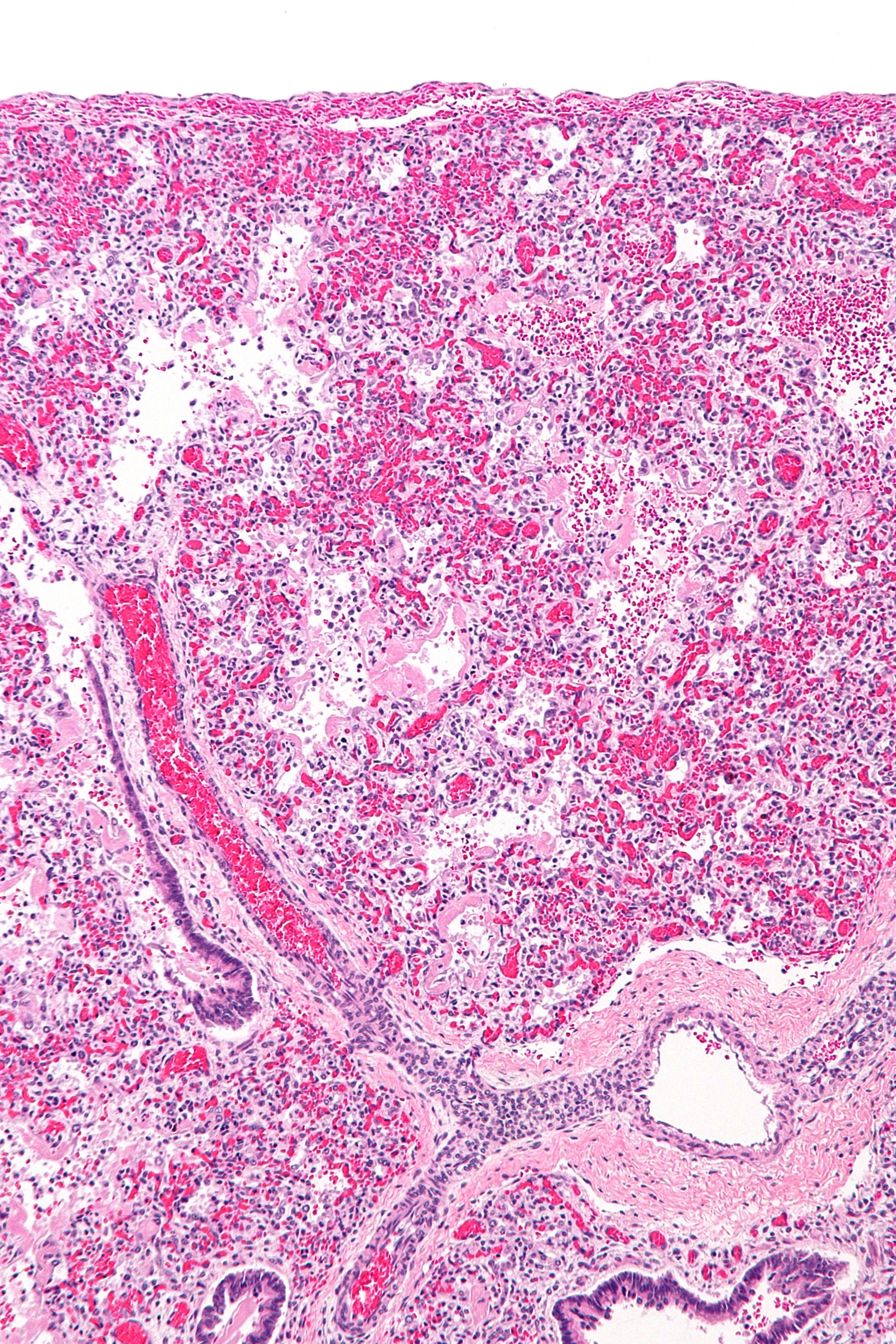
Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) is a type of respiratory failure characterized by rapid onset of widespread inflammation in the lungs. Symptoms include shortness of breath (dyspnea), rapid breathing (tachypnea), and bluish skin coloration (cyanosis). For those who survive, a decreased quality of life is common. Causes may include sepsis, pancreatitis, trauma, pneumonia, and aspiration. The underlying mechanism involves diffuse injury to cells which form the barrier of the microscopic air sacs of the lungs, surfactant dysfunction, activation of the immune system, and dysfunction of the body's regulation of blood clotting. In effect, ARDS impairs the lungs' ability to exchange oxygen and carbon dioxide. Adult diagnosis is based on a PaO2/FiO2 ratio (ratio of partial pressure arterial oxygen and fraction of inspired oxygen) of less than 300 mm Hg despite a positive end-expiratory pressure (PEEP) of more than 5 cm H2O. Cardiogenic pulmonary edema, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |