|
Moisture Sorption Isotherm
At equilibrium, the relationship between water content and equilibrium relative humidity of a material can be displayed graphically by a curve, the so-called moisture sorption isotherm. For each humidity value, a sorption isotherm indicates the corresponding water content value at a given, constant temperature. If the composition or quality of the material changes, then its sorption behaviour also changes. Because of the complexity of sorption process the isotherms cannot be determined explicitly by calculation, but must be recorded experimentally for each product. The relationship between water content and water activity (aw) is complex. An increase in aw is usually accompanied by an increase in water content, but in a non-linear fashion. This relationship between water activity and moisture content at a given temperature is called the moisture sorption isotherm. These curves are determined experimentally and constitute the fingerprint of a food system. BET theory (Brunauer-Emm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
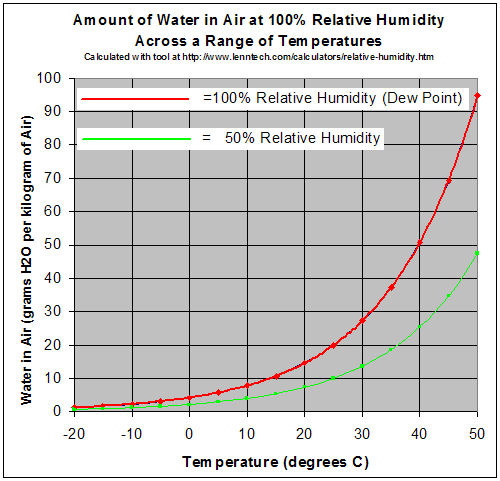
Humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present. Humidity depends on the temperature and pressure of the system of interest. The same amount of water vapor results in higher relative humidity in cool air than warm air. A related parameter is the dew point. The amount of water vapor needed to achieve saturation increases as the temperature increases. As the temperature of a parcel of air decreases it will eventually reach the saturation point without adding or losing water mass. The amount of water vapor contained within a parcel of air can vary significantly. For example, a parcel of air near saturation may contain 28 g of water per cubic metre of air at , but only 8 g of water per cubic metre of air at . Three primary measurements of humidity are widely employed: absolute, relative, and specific. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sorption Isotherm
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a ''surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term ''sorption'' encompasses both processes, while ''desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Content
Water content or moisture content is the quantity of water contained in a material, such as soil (called soil moisture), rock, ceramics, crops, or wood. Water content is used in a wide range of scientific and technical areas, and is expressed as a ratio, which can range from 0 (completely dry) to the value of the materials' porosity at saturation. It can be given on a volumetric or mass (gravimetric) basis. Definitions Volumetric water content, θ, is defined mathematically as: :\theta = \frac where V_w is the volume of water and V_\text = V_s + V_w + V_a is equal to the total volume of the wet material, i.e. of the sum of the volume of solid host material (e.g., soil particles, vegetation tissue) V_s, of water V_w, and of air V_a. Gravimetric water content is expressed by mass (weight) as follows: :u = \frac where m_w is the mass of water and m_s is the mass of the solids. For materials that change in volume with water content, such as coal, the gravimetric water content, ''u' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Activity
Water activity (''aw'') is the partial pressure, partial vapor pressure of water in a solution divided by the standard state partial vapor pressure of water. In the field of food science, the standard state is most often defined as pure water at the same temperature. Using this particular definition, pure distilled water has a water activity of exactly one. Water activity is the thermodynamic activity of water as solvent and the relative humidity of the surrounding air after equilibration. As temperature increases, ''aw'' typically increases, except in some products with crystalline salt or sugar. Water migrates from areas of high ''aw'' to areas of low ''aw''. For example, if honey (''aw'' ≈ 0.6) is exposed to humid air (''aw'' ≈ 0.7), the honey hygroscopy, absorbs water from the air. If salami (''aw'' ≈ 0.87) is exposed to dry air (''aw'' ≈ 0.5), the salami desiccation, dries out, which could drying (food), preserve it or staling, spoil it. Lower ''aw'' substances tend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food System
The term food system describes the interconnected systems and processes that influence nutrition, food, health, community development, and agriculture. A food system includes all processes and infrastructure involved in feeding a population: growing, harvesting, processing, packaging, transporting, marketing, consumption, distribution, and disposal of food and food-related items. It also includes the inputs needed and outputs generated at each of these steps. Food systems fall within agri-food systems, which encompass the entire range of actors and their interlinked value-adding activities in the primary production of food and non-food agricultural products, as well as in food storage, aggregation, post-harvest handling, transportation, processing, distribution, marketing, disposal, and consumption. A food system operates within and is influenced by social, political, economic, and environmental contexts. It also requires human resources that provide labor, research and education. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BET Theory
Brunauer–Emmett–Teller (BET) theory aims to explain the physical adsorption Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which ... of gas molecules on a solid Surface science, surface and serves as the basis for an important analysis technique for the measurement of the specific surface area of materials. The observations are very often referred to as physical adsorption or physisorption. In 1938, Stephen Brunauer, Paul H. Emmett, Paul Hugh Emmett, and Edward Teller presented their theory in the ''Journal of the American Chemical Society''. BET theory applies to systems of multilayer adsorption that usually utilizes a probing gas (called the adsorbate) that do not react chemically with the adsorptive (the material upon which the gas attaches to and the gas phase is called the adsorp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stephen Brunauer
Stephen Brunauer (February 12, 1903 – July 6, 1986) was an American research chemist, government scientist, and university teacher. He resigned from his position with the U.S. Navy during the McCarthy era, when he found it impossible to refute anonymous charges that he was disloyal to the U.S. Early years Stephen Brunauer was born István Brunauer on February 12, 1903, to a Jewish family in Budapest, Hungary. His father was blind and his mother worked as a seamstress. He emigrated to the United States in 1921 and attended City College of New York and Columbia University, majoring in English and chemistry. He received his A.B. from Columbia in 1925. He pursued graduate studies in chemistry and engineering, earning his master's degree in 1929 from George Washington University, where he was a student of Edward Teller, who later described his confidence in asserting his theories and challenging his teachers. While a student, he belonged briefly to the Young Workers' League, a Communis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Desiccant
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccants for specialized purposes may be in forms other than solid, and may work through other principles, such as chemical bonding of water molecules. They are commonly encountered in foods to retain crispness. Industrially, desiccants are widely used to control the level of water in gas streams. Types of desiccants Although some desiccants are chemically inert, others are extremely reactive and require specialized handling techniques. The most common desiccant is silica gel, an otherwise inert, nontoxic, water-insoluble white solid. Tens of thousands of tons are produced annually for this purpose. Other common desiccants include activated charcoal, calcium sulfate, calcium chloride, and molecular sieves (typically, zeolites). Desicca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Chemistry
Food chemistry is the study of chemical processes and interactions of all biological and non-biological components of foods. The biological substances include such items as meat, poultry, lettuce, beer, milk as examples. It is similar to biochemistry in its main components such as carbohydrates, lipids, and protein, but it also includes areas such as water, vitamins, minerals, enzymes, food additives, flavors, and colors. This discipline also encompasses how products change under certain food processing techniques and ways either to enhance or to prevent them from happening. An example of enhancing a process would be to encourage fermentation of dairy products with microorganisms that convert lactose to lactic acid; an example of preventing a process would be stopping the browning on the surface of freshly cut apples using lemon juice or other acidulated water. History of food chemistry The scientific approach to food and nutrition arose with attention to agricultural chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |