|
Microstate (statistical Mechanics)
In statistical mechanics, a microstate is a specific microscopic configuration of a thermodynamic system that the system may occupy with a certain probability in the course of its thermal fluctuations. In contrast, the macrostate of a system refers to its macroscopic properties, such as its temperature, pressure, volume and density. Treatments on statistical mechanics define a macrostate as follows: a particular set of values of energy, the number of particles, and the volume of an isolated thermodynamic system is said to specify a particular macrostate of it. In this description, microstates appear as different possible ways the system can achieve a particular macrostate. A macrostate is characterized by a probability distribution of possible states across a certain statistical ensemble of all microstates. This distribution describes the probability of finding the system in a certain microstate. In the thermodynamic limit, the microstates visited by a macroscopic system during ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canonical Ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat bath, so that the states of the system will differ in total energy. The principal thermodynamic variable of the canonical ensemble, determining the probability distribution of states, is the absolute temperature (symbol: ). The ensemble typically also depends on mechanical variables such as the number of particles in the system (symbol: ) and the system's volume (symbol: ), each of which influence the nature of the system's internal states. An ensemble with these three parameters is sometimes called the ensemble. The canonical ensemble assigns a probability to each distinct microstate given by the following exponential: :P = e^, where is the total energy of the microstate, and is the Boltzmann constant. The number is the free ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indicator Function
In mathematics, an indicator function or a characteristic function of a subset of a set is a function that maps elements of the subset to one, and all other elements to zero. That is, if is a subset of some set , one has \mathbf_(x)=1 if x\in A, and \mathbf_(x)=0 otherwise, where \mathbf_A is a common notation for the indicator function. Other common notations are I_A, and \chi_A. The indicator function of is the Iverson bracket of the property of belonging to ; that is, :\mathbf_(x)= \in A For example, the Dirichlet function is the indicator function of the rational numbers as a subset of the real numbers. Definition The indicator function of a subset of a set is a function \mathbf_A \colon X \to \ defined as \mathbf_A(x) := \begin 1 ~&\text~ x \in A~, \\ 0 ~&\text~ x \notin A~. \end The Iverson bracket provides the equivalent notation, \in A/math> or to be used instead of \mathbf_(x)\,. The function \mathbf_A is sometimes denoted , , , or even just . Nota ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generalized Coordinates
In analytical mechanics, generalized coordinates are a set of parameters used to represent the state of a system in a configuration space. These parameters must uniquely define the configuration of the system relative to a reference state.,p. 397, §7.2.1 Selection of generalized coordinates/ref> The generalized velocities are the time derivatives of the generalized coordinates of the system. The adjective "generalized" distinguishes these parameters from the traditional use of the term "coordinate" to refer to Cartesian coordinates An example of a generalized coordinate would be to describe the position of a pendulum using the angle of the pendulum relative to vertical, rather than by the x and y position of the pendulum. Although there may be many possible choices for generalized coordinates for a physical system, they are generally selected to simplify calculations, such as the solution of the equations of motion for the system. If the coordinates are independent of o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
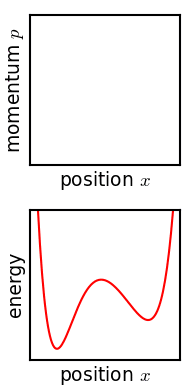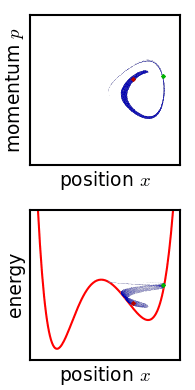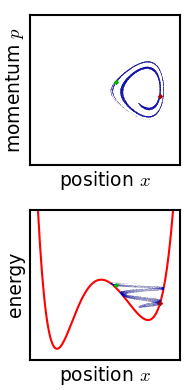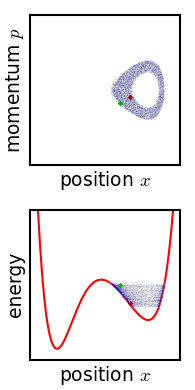
Phase Space
In dynamical system theory, a phase space is a space in which all possible states of a system are represented, with each possible state corresponding to one unique point in the phase space. For mechanical systems, the phase space usually consists of all possible values of position and momentum variables. It is the outer product of direct space and reciprocal space. The concept of phase space was developed in the late 19th century by Ludwig Boltzmann, Henri Poincaré, and Josiah Willard Gibbs. Introduction In a phase space, every degree of freedom or parameter of the system is represented as an axis of a multidimensional space; a one-dimensional system is called a phase line, while a two-dimensional system is called a phase plane. For every possible state of the system or allowed combination of values of the system's parameters, a point is included in the multidimensional space. The system's evolving state over time traces a path (a phase-space trajectory for the system) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Degrees Of Freedom (physics And Chemistry)
In physics and chemistry, a degree of freedom is an independent physical parameter in the formal description of the state of a physical system. The set of all states of a system is known as the system's phase space, and the degrees of freedom of the system are the dimensions of the phase space. The location of a particle in three-dimensional space requires three position coordinates. Similarly, the direction and speed at which a particle moves can be described in terms of three velocity components, each in reference to the three dimensions of space. If the time evolution of the system is deterministic (where the state at one instant uniquely determines its past and future position and velocity as a function of time) such a system has six degrees of freedom. If the motion of the particle is constrained to a lower number of dimensions – for example, the particle must move along a wire or on a fixed surface – then the system has fewer than six degrees of freedom. On the oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Theorem
The adiabatic theorem is a concept in quantum mechanics. Its original form, due to Max Born and Vladimir Fock (1928), was stated as follows: :''A physical system remains in its instantaneous eigenstate if a given perturbation is acting on it slowly enough and if there is a gap between the eigenvalue and the rest of the Hamiltonian's spectrum.'' In simpler terms, a quantum mechanical system subjected to gradually changing external conditions adapts its functional form, but when subjected to rapidly varying conditions there is insufficient time for the functional form to adapt, so the spatial probability density remains unchanged. Diabatic vs. adiabatic processes At some initial time t_0 a quantum-mechanical system has an energy given by the Hamiltonian \hat(t_0); the system is in an eigenstate of \hat(t_0) labelled \psi(x,t_0). Changing conditions modify the Hamiltonian in a continuous manner, resulting in a final Hamiltonian \hat(t_1) at some later time t_1. The system will e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (thermodynamics)
In thermodynamics, work is one of the principal processes by which a thermodynamic system can interact with its surroundings and exchange energy. An exchange of energy is facilitated by a mechanism through which the system can spontaneously exert macroscopic forces on its surroundings, or vice versa. In the surroundings, this mechanical work can lift a weight, for example. The externally measured forces and external effects may be electromagnetic,Guggenheim, E.A. (1985). ''Thermodynamics. An Advanced Treatment for Chemists and Physicists'', seventh edition, North Holland, Amsterdam, .Jackson, J.D. (1975). ''Classical Electrodynamics'', second edition, John Wiley and Sons, New York, .Konopinski, E.J. (1981). ''Electromagnetic Fields and Relativistic Particles'', McGraw-Hill, New York, . gravitational,North, G.R., Erukhimova, T.L. (2009). ''Atmospheric Thermodynamics. Elementary Physics and Chemistry'', Cambridge University Press, Cambridge (UK), . or mechanical (such as pressure-v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat
In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is also often used to refer to the thermal energy contained in a system as a component of its internal energy and that is reflected in the temperature of the system. For both uses of the term, heat is a form of energy. An example of formal vs. informal usage may be obtained from the right-hand photo, in which the metal bar is "conducting heat" from its hot end to its cold end, but if the metal bar is considered a thermodynamic system, then the energy flowing within the metal bar is called internal energy, not heat. The hot metal bar is also transferring heat to its surroundings, a correct statement for both the strict and loose meanings of ''heat''. Another example of informal usage is the term '' heat content'', used despite the fact that p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Third Law Of Thermodynamics
The third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: This constant value cannot depend on any other parameters characterizing the closed system, such as pressure or applied magnetic field. At absolute zero (zero kelvins) the system must be in a state with the minimum possible energy. Entropy is related to the number of accessible microstates, and there is typically one unique state (called the ground state) with minimum energy. In such a case, the entropy at absolute zero will be exactly zero. If the system does not have a well-defined order (if its order is glassy, for example), then there may remain some finite entropy as the system is brought to very low temperatures, either because the system becomes locked into a configuration with non-minimal energy or because the minimum energy state is non-unique. The constant value is called the residual entropy of the system. The entropy is essentially a state-function meaning t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Law Of Thermodynamics
The second law of thermodynamics is a physical law based on universal experience concerning heat and Energy transformation, energy interconversions. One simple statement of the law is that heat always moves from hotter objects to colder objects (or "downhill"), unless energy in some form is supplied to reverse the direction of heat flow. Another definition is: "Not all heat energy can be converted into Work (thermodynamics), work in a cyclic process."Young, H. D; Freedman, R. A. (2004). ''University Physics'', 11th edition. Pearson. p. 764. The second law of thermodynamics in other versions establishes the concept of entropy as a physical property of a thermodynamic system. It can be used to predict whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics and provides necessary criteria for spontaneous processes. The second law may be formulated by the observation that the entropy of isolated systems ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ludwig Boltzmann
Ludwig Eduard Boltzmann (; 20 February 1844 – 5 September 1906) was an Austrian physicist and philosopher. His greatest achievements were the development of statistical mechanics, and the statistical explanation of the second law of thermodynamics. In 1877 he provided the current definition of entropy, S = k_ \ln \Omega \!, where Ω is the number of microstates whose energy equals the system's energy, interpreted as a measure of statistical disorder of a system. Max Planck named the constant the Boltzmann constant. Statistical mechanics is one of the pillars of modern physics. It describes how macroscopic observations (such as temperature and pressure) are related to microscopic parameters that fluctuate around an average. It connects thermodynamic quantities (such as heat capacity) to microscopic behavior, whereas, in classical thermodynamics, the only available option would be to measure and tabulate such quantities for various materials. Biography Childhood and educatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)



