|
Metal–insulator Transition
Metal–insulator transitions are transitions of a material from a metal (material with good electrical conductivity of electric charges) to an insulator (material where conductivity of charges is quickly suppressed). These transitions can be achieved by tuning various ambient parameters such as temperature, pressure or, in case of a semiconductor, doping. History The basic distinction between metals and insulators was proposed by Bethe, Sommerfeld and Bloch in 1928/1929. It distinguished between conducting metals (with partially filled bands) and nonconducting insulators. However, in 1937 de Boer and Evert Verwey reported that many transition-metal oxides (such as NiO) with a partially filled d-band were poor conductors, often insulating. In the same year, the importance of the electron-electron correlation was stated by Peierls. Since then, these materials as well as others exhibiting a transition between a metal and an insulator have been extensively studied, e.g. by Si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mott Insulator
Mott insulators are a class of materials that are expected to conduct electricity according to conventional band theories, but turn out to be insulators (particularly at low temperatures). These insulators fail to be correctly described by band theories of solids due to their strong electron–electron interactions, which are not considered in conventional band theory. A Mott transition is a transition from a metal to an insulator, driven by the strong interactions between electrons. One of the simplest models that can capture Mott transition is the Hubbard model. The band gap in a Mott insulator exists between bands of like character, such as 3d electron bands, whereas the band gap in charge-transfer insulators exists between anion and cation states, such as between O 2p and Ni 3d bands in NiO. History Although the band theory of solids had been very successful in describing various electrical properties of materials, in 1937 Jan Hendrik de Boer and Evert Johannes Willem Ve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Refractivity
Molar refractivity,W. Foerst et.al. ''Chemie für Labor und Betrieb'', 1967, ''3'', 32-34. https://organic-btc-ilmenau.jimdo.com/app/download/9062135220/molrefraktion.pdf?t=1616948905 A, is a measure of the total polarizability of a mole of a substance and is dependent on the temperature, the index of refraction, and the pressure. The molar refractivity is defined as : A = \frac N_A \alpha, where N_A \approx 6.022 \times 10^ is the Avogadro constant and \alpha is the mean polarizability of a molecule. Substituting the molar refractivity into the Lorentz-Lorenz formula gives, for gasses : A = \frac \frac where n is the refractive index, p is the pressure of the gas, R is the universal gas constant, and T is the (absolute) temperature. For a gas, n^2 \approx 1, so the molar refractivity can be approximated by :A = \frac \frac. In SI units, R has units of J mol−1 K−1, T has units K, n has no units, and p has units of Pa, so the units of A are m3 mol−1. In terms of density ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blue Bronze
In chemistry, molybdenum bronze is a generic name for certain mixed oxides of molybdenum with the generic formula where A may be hydrogen, an alkali metal cation (such as Li+, Na+, K+), and Tl+. These compounds form deeply coloured plate-like crystals with a metallic sheen, hence their name. These bronzes derive their metallic character from partially occupied 4d bands. The oxidation states in K0.28MoO3 are K+1, O2−, and Mo+5.72. MoO3 is an insulator, with an unfilled 4d band. These compounds have been much studied since the 1980s due to their markedly anisotropic electrical properties, reflecting their layered structure. The electrical resistivity can vary considerably depending on the direction, in some cases by 200:1 or more. They are generally non-stoichiometric compounds. Some are metals and some are semiconductors. Preparation The first report of a "molybdenum bronze" was by Alfred Stavenhagen and E. Engels in 1895. They reported that electrolysis of molten a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
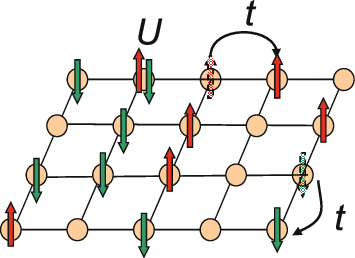
Hubbard Model
The Hubbard model is an approximate model used to describe the transition between conducting and insulating systems. It is particularly useful in solid-state physics. The model is named for John Hubbard. The Hubbard model states that each electron experiences competing forces: one pushes it to tunnel to neighboring atoms, while the other pushes it away from its neighbors. Its Hamiltonian thus has two terms: a kinetic term allowing for tunneling ("hopping") of particles between lattice sites and a potential term reflecting on-site interaction. The particles can either be fermions, as in Hubbard's original work, or bosons, in which case the model is referred to as the "Bose–Hubbard model". The Hubbard model is a useful approximation for particles in a periodic potential at sufficiently low temperatures, where all the particles may be assumed to be in the lowest Bloch band, and long-range interactions between the particles can be ignored. If interactions between particles at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin (physics)
Spin is a conserved quantity carried by elementary particles, and thus by composite particles (hadrons) and atomic nucleus, atomic nuclei. Spin is one of two types of angular momentum in quantum mechanics, the other being ''orbital angular momentum''. The orbital angular momentum operator is the quantum-mechanical counterpart to the classical angular momentum of orbital revolution and appears when there is periodic structure to its wavefunction as the angle varies. For photons, spin is the quantum-mechanical counterpart of the Polarization (waves), polarization of light; for electrons, the spin has no classical counterpart. The existence of electron spin angular momentum is inferred from experiments, such as the Stern–Gerlach experiment, in which silver atoms were observed to possess two possible discrete angular momenta despite having no orbital angular momentum. The existence of the electron spin can also be inferred theoretically from the spin–statistics theorem and from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Correlation
Electronic correlation is the interaction between electrons in the electronic structure of a quantum system. The correlation energy is a measure of how much the movement of one electron is influenced by the presence of all other electrons. Atomic and molecular systems Within the Hartree–Fock method of quantum chemistry, the antisymmetric wave function is approximated by a single Slater determinant. Exact wave functions, however, cannot generally be expressed as single determinants. The single-determinant approximation does not take into account Coulomb correlation, leading to a total electronic energy different from the exact solution of the non-relativistic Schrödinger equation within the Born–Oppenheimer approximation. Therefore, the Hartree–Fock limit is always above this exact energy. The difference is called the ''correlation energy'', a term coined by Löwdin. The concept of the correlation energy was studied earlier by Wigner. A certain amount of electron c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conduction Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantization of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics, a band gap, also called an energy gap, is an energy range in a solid where no electronic states can exist. In graphs of the electronic band structure of solids, the band gap generally refers to the energy difference (in electron volts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote a valence electron bound to an atom to become a conduction electron, which is free to move within the crystal lattice and serve as a charge carrier to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there is no generated current due to no net charge carrier mobility. However, if some electrons transfer from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Level
The Fermi level of a solid-state body is the thermodynamic work required to add one electron to the body. It is a thermodynamic quantity usually denoted by ''µ'' or ''E''F for brevity. The Fermi level does not include the work required to remove the electron from wherever it came from. A precise understanding of the Fermi level—how it relates to electronic band structure in determining electronic properties, how it relates to the voltage and flow of charge in an electronic circuit—is essential to an understanding of solid-state physics. In band structure theory, used in solid state physics to analyze the energy levels in a solid, the Fermi level can be considered to be a hypothetical energy level of an electron, such that at thermodynamic equilibrium this energy level would have a ''50% probability of being occupied at any given time''. The position of the Fermi level in relation to the band energy levels is a crucial factor in determining electrical properties. The Fermi le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Band Structure
In solid-state physics, the electronic band structure (or simply band structure) of a solid describes the range of energy levels that electrons may have within it, as well as the ranges of energy that they may not have (called ''band gaps'' or ''forbidden bands''). Band theory derives these bands and band gaps by examining the allowed quantum mechanical wave functions for an electron in a large, periodic lattice of atoms or molecules. Band theory has been successfully used to explain many physical properties of solids, such as electrical resistivity and optical absorption, and forms the foundation of the understanding of all solid-state devices (transistors, solar cells, etc.). Why bands and band gaps occur The electrons of a single, isolated atom occupy atomic orbitals each of which has a discrete energy level. When two or more atoms join together to form a molecule, their atomic orbitals overlap and hybridize. Similarly, if a large number ''N'' of identical atoms come ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula Fe2+Fe3+2O4. It is one of the oxides of iron, and is ferrimagnetic; it is attracted to a magnet and can be magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism. Magnetite is black or brownish-black with a metallic luster, has a Mohs hardness of 5–6 and leaves a black streak. Small grains of magnetite are very common in igneous and metamorphic rocks. The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ''ferrous-ferric oxide''. Properties In addition to igneous rocks, magnetite also occurs in sedimentary rocks, including banded iron formations and in lake and marine sediments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |