|
M2 Hol Family
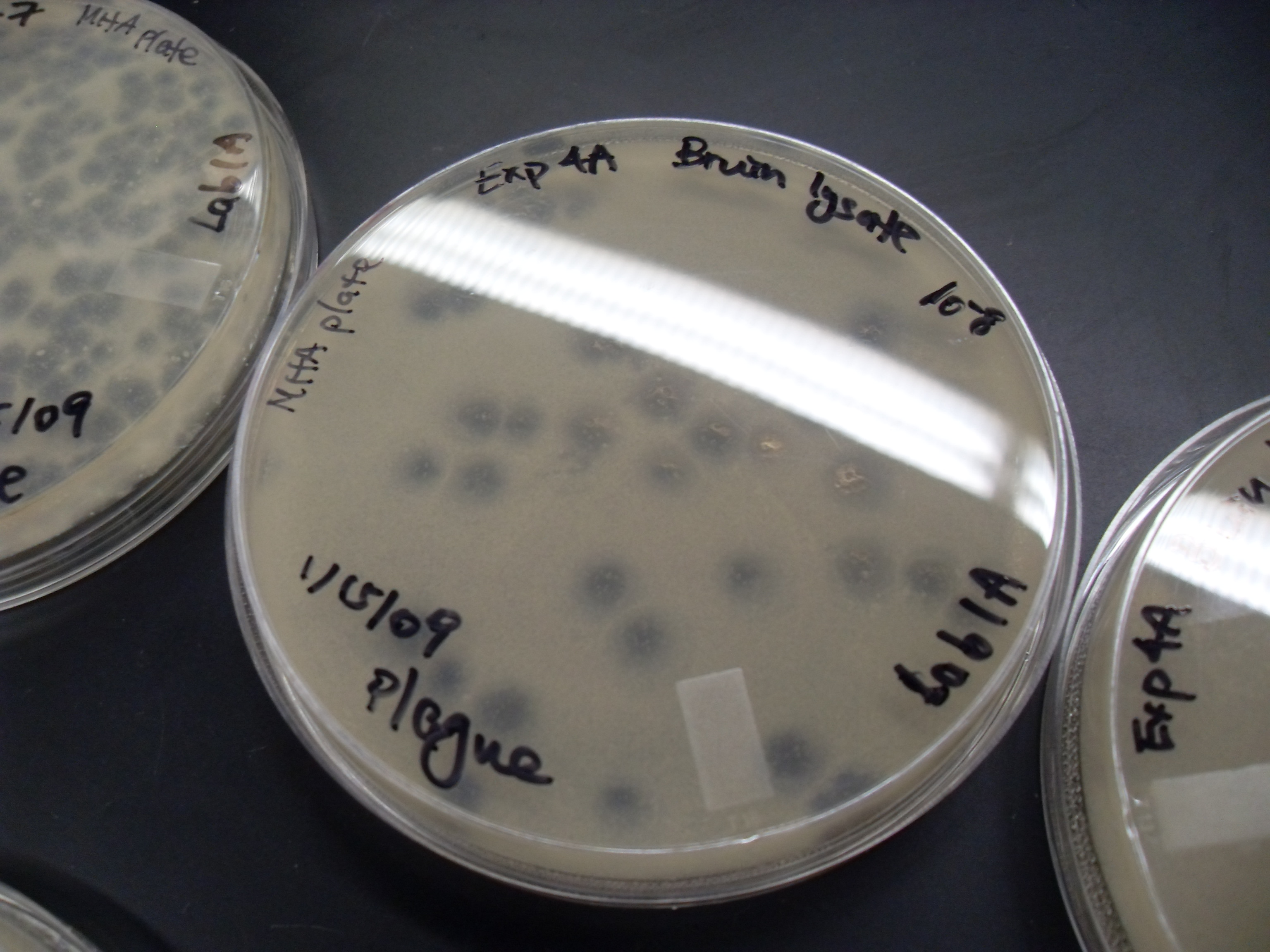
The ''Mycobacterial'' 2 TMS Phage Holin (M2 Hol) FamilyTC# 1.E.36 is a group of transporters belonging to the Holin Superfamily VII. The ''Mycobactrerial'' 2 transmembrane segment (TMS) Holins have been identified and recognized by Catalao et al (2012). The ''Mycobacterium'' phage D29 gp11 proteinTC# 1.E.36.1.7 is a holin that, upon expression, rapidly kills both ''E. coli'' and '' Mycobacterium smegmatis''. Shortening gp11 from its C-terminus resulted in diminished cytotoxicity and smaller holes. The two TMSs at the N-terminus alone do not integrate into the cytoplasmic membrane and do not show toxicity. Fusion of the two TMSs and a small C-terminal coiled-coil region resulted in restoration of cell killing. The second TMS is dispensable for toxicity. The gp11 C-terminal region is therefore necessary but not sufficient for toxicity. See also * Holin * Lysin Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holin Superfamily VII
The Holin superfamily VII is a superfamily of integral membrane transport proteins. It is one of the seven different holin superfamilies in total. In general, these proteins are thought to play a role in regulated cell death, although functionality varies between families and individual members. The Holin superfamily VII includes one TC family: 1.E.36- The Mycobacterial 2 TMS Phage Holin (M2 Hol) Family Superfamily VII consists of familTC# 1.E.36 which has six subfamilies, all distantly related to each other. Reddy and Saier (2013) found that there was also some indication that this family could possibly be related to the SPP1 Holin familyTC# 1.E.31 and the 2/3 Holin familyTC# 1.E.33. These three families share the characteristics that they consist of members that are of about the same sizes and have 2 transmembrane segments (TMSs). There is however an exception; subfamily 6TC# 1.E.36.6 includes proteins displaying 4 putative TMSs. See also * Holin * Lysin Lysins, also known ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycobacterium
''Mycobacterium'' is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis ('' M. tuberculosis'') and leprosy ('' M. leprae'') in humans. The Greek prefix ''myco-'' means 'fungus', alluding to this genus' mold-like colony surfaces. Since this genus has cell walls with Gram-positive and Gram-negative features, acid-fast staining is used to emphasize their resistance to acids, compared to other cell types. Metabolism and Morphology Mycobacteria are aerobic with 0.2-0.6 µm wide and 1.0-10 µm long rod shapes. They are generally non-motile, except for the species ''Mycobacterium marinum'', which has been shown to be motile within macrophages. Mycobacteria possess capsules and most do not form endospores. ''M. marinum'' and perhaps ''M. bovis'' have been shown to sporulate; however, this has been contested by further research. The disti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycobacterium Smegmatis
''Mycobacterium smegmatis'' is an acid-fast bacterial species in the phylum ''Actinomycetota'' and the genus ''Mycobacterium''. It is 3.0 to 5.0 µm long with a bacillus shape and can be stained by Ziehl–Neelsen method and the auramine-rhodamine fluorescent method. It was first reported in November 1884 by Lustgarten, who found a bacillus with the staining appearance of tubercle bacilli in syphilitic chancres. Subsequent to this, Alvarez and Tavel found organisms similar to that described by Lustgarten also in normal genital secretions (smegma). This organism was later named ''M. smegmatis''. Some species of the genus ''Mycobacterium'' have recently been renamed to ''Mycolicibacterium'', so that ''M. smegmatis'' is now ''Mycolicibacterium smegmatis''. Virulence ''M. smegmatis'' is generally considered a non-pathogenic microorganism; however, in some very rare cases, it may cause disease. Use in research ''M. smegmatis'' is useful for the research analysis of other ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxicity
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a change i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coiled Coil
A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope. (Dimers and trimers are the most common types.) Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where Crick worked. Pauling and Crick met and spoke about various topics; at one point, Crick asked whether Pauling had considered "coiled coils" (Crick came up with the term), to which Pauling said he had. Upon returning to the United States, Pauling resumed research on the topic. He conc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holin
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins to reach and degrade peptidoglycan, a component of bacterial cell walls. Holins have been shown to regulate the timing of lysis with great precision. Over 50 unrelated gene families encode holins, making them the most diverse group of proteins with common function. Together with lysins, holins are being studied for their potential use as antibacterial agents. While canonical holins act by forming large pores, pinholins such as the S protein of lambdoid phage 21 act by forming heptameric channels that depolarize the bacterial membrane. They are associated with SAR endolysins, which remain inactive in the periplasm prior to the depolarization of the membrane. Viruses that infect eukaryotic cells may use similar channel-forming proteins called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysin
Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins. Lysins are being used as antibacterial agents due to their high effectiveness and specificity in comparison with antibiotics, which are susceptible to bacterial resistance. Structure Double-stranded DNA phage lysins tend to lie within the 25 to 40 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holins
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins to reach and degrade peptidoglycan, a component of bacterial cell walls. Holins have been shown to regulate the timing of lysis with great precision. Over 50 unrelated gene families encode holins, making them the most diverse group of proteins with common function. Together with lysins, holins are being studied for their potential use as antibacterial agents. While canonical holins act by forming large pores, pinholins such as the S protein of lambdoid phage 21 act by forming heptameric channels that depolarize the bacterial membrane. They are associated with SAR endolysins, which remain inactive in the periplasm prior to the depolarization of the membrane. Viruses that infect eukaryotic cells may use similar channel-forming proteins calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |