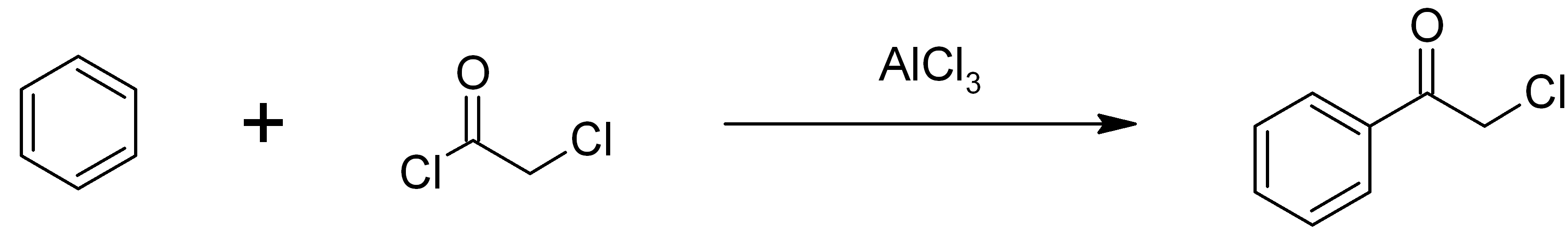|
M1 Frangible Grenade
The Frangible Grenade M1 was a specially designed factory produced molotov cocktail created by the United States in 1942 as it entered World War II (1939–1945). It was designed to provide lightly armed personnel (self-defense militias, soldiers, commandos, and Allied partisans) with simple, uncomplicated weapons that were easy to mass-produce. It provided a cheap stopgap means of knocking out enemy vehicles, clearing out strongpoints, and harassing or killing enemy personnel until more effective weapons could be produced and distributed. It was dubbed " frangible" because it was made from glass, which is brittle and easily broken. History In late December 1941 the United States entered into World War II with an unprepared military, low stocks of arms and munitions, and fears of attack or invasion by the Axis Powers. To counter this looming threat, a series of Molotov cocktail-style devices, nominally designated as "grenades", were developed in early 1942. It consisted of a pint- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Department Of War
The United States Department of War, also called the War Department (and occasionally War Office in the early years), was the United States Cabinet department originally responsible for the operation and maintenance of the United States Army, also bearing responsibility for naval affairs until the establishment of the Navy Department in 1798, and for most land-based air forces until the creation of the Department of the Air Force on September 18, 1947. The Secretary of War, a civilian with such responsibilities as finance and purchases and a minor role in directing military affairs, headed the War Department throughout its existence. The War Department existed from August 7, 1789 until September 18, 1947, when it split into the Department of the Army and the Department of the Air Force. The Department of the Army and Department of the Air Force later joined the Department of the Navy under the United States Department of Defense in 1949. History 18th century The Depar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Agent
A blood agent is a toxic chemical agent that affects the body by being absorbed into the blood. Blood agents are fast-acting, potentially lethal poisons that typically manifest at room temperature as volatile colorless gases with a faint odor. They are either cyanide- or arsenic-based. Exposure Blood agents work through inhalation or ingestion. As chemical weapons, blood agents are typically disseminated as aerosols and take effect through inhalation. Due to their volatility, they are more toxic in confined areas than in open areas. Cyanide compounds occur in small amounts in the natural environment and in cigarette smoke. They are also used in several industrial processes and as pesticides. Cyanides are released when synthetic fabrics or polyurethane burn, and may thus contribute to fire-related deaths.Walsh, 151. Arsine gas, formed when arsenic encounters an acid, is used as a pesticide and in the semiconductor industry; most exposures to it occur accidentally in the work ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vesicant
A blister agent (or vesicant), is a chemical compound that causes severe skin, eye and mucosal pain and irritation. They are named for their ability to cause severe chemical burns, resulting in painful water blisters on the bodies of those affected. Although the term is often used in connection with large-scale burns caused by chemical spills or chemical warfare agents, some naturally occurring substances such as are also blister-producing agents (vesicants). [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geraniums
''Pelargonium'' () is a genus of flowering plants that includes about 280 species of perennials, succulents, and shrubs, commonly called geraniums, pelargoniums, or storksbills. ''Geranium'' is also the botanical name and common name of a separate genus of related plants, also known as cranesbills. Both genera belong to the family Geraniaceae. Carl Linnaeus originally included all the species in one genus, ''Geranium'', and they were later separated into two genera by Charles Louis L'Héritier de Brutelle in 1789. While ''Geranium'' species are mostly temperate herbaceous plants, dying down in winter, ''Pelargonium'' species are evergreen perennials indigenous to warm temperate and tropical regions of the world, with many species in southern Africa. They are drought and heat tolerant, but can tolerate only minor frosts. Some species are extremely popular garden plants, grown as houseplants and bedding plants in temperate regions. They have a long flowering period, with flowers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewisite
Lewisite (L) (A-243) is an organoarsenic compound. It was once manufactured in the U.S., Japan, Germany and the Soviet Union for use as a Chemical warfare, chemical weapon, acting as a vesicant (blister agent) and lung irritant. Although the substance is colorless and odorless in its pure form, impure samples of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a distinctive odor that has been described as similar to Pelargonium, geraniums. Chemical reactions The compound is prepared by the addition of arsenic trichloride to acetylene in the presence of a suitable catalyst: :AsCl3 + C2H2 → ClCHCHAsCl2 (Lewisite) Lewisite, like other arsenous chlorides, hydrolysis, hydrolyses in water to form hydrochloric acid and chlorovinylarsenous oxide (a less-powerful blister agent): :ClCHCHAsCl2 + 2 H2O → ClCHCHAs(OH)2 + 2 HCl This reaction is accelerated in alkaline solutions, and forms acetylene and trisodium arsenate. Lewisite reacts with metals to form hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorosulfuric Acid
Chlorosulfuric acid (IUPAC name: sulfurochloridic acid) is the inorganic compound with the formula HSO3Cl. It is also known as chlorosulfonic acid, being the sulfonic acid of chlorine. It is a distillable, colorless liquid which is hygroscopic and a powerful lachrymator. Commercial samples usually are pale brown or straw colored. Salts and esters of chlorosulfuric acid are known as chlorosulfates. Structure and properties Chlorosulfuric acid is a tetrahedral molecule. The formula is more descriptively written SO2(OH)Cl, but HSO3Cl is traditional. It is an intermediate, chemically and conceptually, between sulfuryl chloride (SO2Cl2) and sulfuric acid (H2SO4). The compound is rarely obtained pure. Upon standing with excess sulfur trioxide, it decomposes to pyrosulfuryl chlorides: :2 ClSO3H + SO3 → H2SO4 + S2O5Cl2 Synthesis The industrial synthesis entails the reaction of hydrogen chloride with a solution of sulfur trioxide in sulfuric acid: :HCl + SO3 → ClSO3H It c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Trioxide
Sulfur trioxide (alternative spelling sulphur trioxide, also known as ''nisso sulfan'') is the chemical compound with the formula SO3. It has been described as "unquestionably the most important economically" sulfur oxide. It is prepared on an industrial scale as a precursor to sulfuric acid. Sulfur trioxide exists in several forms - gaseous monomer, crystalline trimer, and solid polymer. Sulfur trioxide is a solid at just below room temperature with a relatively narrow liquid range. Gaseous SO3 is the primary precursor to acid rain. Molecular structure and bonding Monomer The molecule SO3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D3h point group. The sulfur atom has an oxidation state of +6 and may be assigned a formal charge value as low as 0 (if all three sulfur-oxygen bonds are assumed to be double bonds) or as high as +2 (if the Octet Rule is assumed). When the formal charge is non-zero, the S-O bonding is assumed to be delocalized. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tear Gas
Tear gas, also known as a lachrymator agent or lachrymator (), sometimes colloquially known as "mace" after the early commercial aerosol, is a chemical weapon that stimulates the nerves of the lacrimal gland in the eye to produce tears. In addition, it can cause severe eye and respiratory pain, skin irritation, bleeding, and blindness. Common lachrymators both currently and formerly used as tear gas include pepper spray (OC gas), PAVA spray ( nonivamide), CS gas, CR gas, CN gas (phenacyl chloride), bromoacetone, xylyl bromide and Mace (a branded mixture). While lachrymatory agents are commonly deployed for riot control by law enforcement and military personnel, its use in warfare is prohibited by various international treaties.E.g. the Geneva Protocol of 1925 prohibited the use of "asphyxiating gas, or any other kind of gas, liquids, substances or similar materials". During World War I, increasingly toxic and deadly lachrymatory agents were used. The short and long ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane, is an organic compound with formula C H Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Structure The molecule adopts a tetrahedral molecular geometry with C3v symmetry. Natural occurrence The total global flux of chloroform through the environment is approximately tonnes per year, and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. Abiotic processes are also believed to contribute to natural chloroform productions in soils although the mechanism is still unclear. Chloroform volatilizes readily from soil and surface water and undergoes degradation in air to produce phosgene, dichloromethane, formyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorpicrin
Chloropicrin, also known as PS and nitrochloroform, is a chemical compound currently used as a broad-spectrum antimicrobial, fungicide, herbicide, insecticide, and nematicide. It was used as a poison gas in World War I. Its chemical structural formula is Cl3CNO2. Synthesis Chloropicrin was discovered in 1848 by Scottish chemist John Stenhouse. He prepared it by the reaction of sodium hypochlorite with picric acid: : HOC6H2(NO2)3 + 11 NaOCl → 3 Cl3CNO2 + 3 Na2CO3 + 3 NaOH + 2 NaCl Because of the precursor used, Stenhouse named the compound chloropicrin, although the two compounds are structurally dissimilar. Today, chloropicrin is manufactured by the reaction of nitromethane with sodium hypochlorite: : H3CNO2 + 3 NaOCl → Cl3CNO2 + 3 NaOH or by the reaction of chloroform with nitric acid: : CHCl3 + HNO3 → CCl3NO2 + H2O Properties Chloropicrin's chemical formula is CCl3NO2 and its molecular weight is 164.38 grams/mole. Pure chloropicrin is a colorless liquid, with a boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenacyl Chloride
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Preparation Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: Riot control agent It was investigated, but not used, during the First and Second World Wars. Because of its significantly greater toxicity, it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “Mace” or tear gas, its use is falling as pepper spray both works and disperses mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Cyanide
Hydrogen cyanide, sometimes called prussic acid, is a chemical compound with the formula HCN and structure . It is a colorless, extremely poisonous, and flammable liquid that boils slightly above room temperature, at . HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. Structure and general properties Hydrogen cyanide is a linear molecule, with a triple bond between carbon and nitrogen. The tautomer of HCN is HNC, hydrogen isocyanide. Hydrogen cyanide is weakly acidic with a p''K''a of 9.2. It partially ionizes in water solution to give the cyanide anion, CN−. A solution of hydrogen cyanide in water, represented as HCN, is called ''hydrocyanic acid''. The salts of the cyan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)