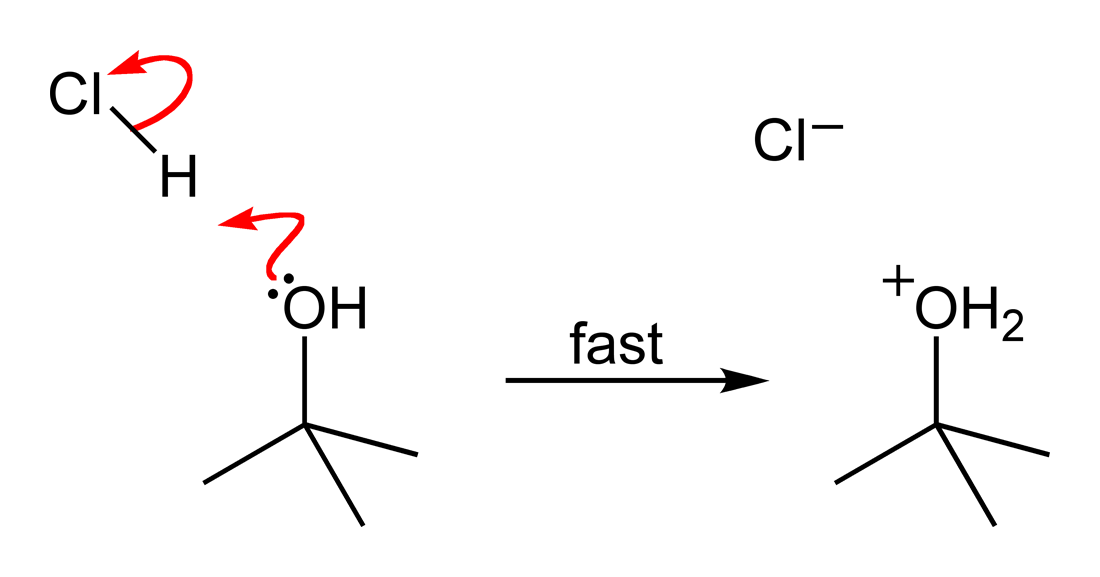|
Living Cationic Polymerization
Living cationic polymerization is a living polymerization technique involving cationic propagating species. It enables the synthesis of very well defined polymers (low molar mass distribution) and of polymers with unusual architecture such as star polymers and block copolymers and living cationic polymerization is therefore as such of commercial and academic interest. Basics In carbocationic polymerization the active site is a carbocation with a counterion in close proximity. The basic reaction steps are: : A+B− + H2C=CHR → A-CH2-RHC+----B− * Chain propagation: : A-CH2-RHC+----B− + H2C=CHR → A-(CH2-RHC)n-CH2-RHC+----B− * Chain termination: : A-(CH2-RHC)n-CH2-RHC+----B− → A-(CH2-RHC)n-CH2-RHC-B * chain transfer: : A-(CH2-RHC)n-CH2-RHC+----B− → A-(CH2-RHC)n-CH2=CR H+B− Living cationic polymerization is characterised by defined and controlled initiation and propagation while minimizing side-reactions termination and chain transfer. Transfer and terminatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Living Polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar (i.e. they have a very low polydispersity index). Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups. Living polymerization is desirable because it offers precision and control in macromolecular synthesis. This is important since many of the novel/useful properties of polymers result from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Trichloride
Boron trichloride is the inorganic compound with the formula BCl3. This colorless gas is a reagent in organic synthesis. It is highly reactive toward water. Production and structure Boron reacts with halogens to give the corresponding trihalides. Boron trichloride is, however, produced industrially by direct chlorination of boron oxide and carbon at 501 °C. :B2O3 + 3 C + 3 Cl2 → 2 BCl3 + 3 CO The carbothermic reaction is analogous to the Kroll process for the conversion of titanium dioxide to titanium tetrachloride. In the laboratory BF3 reacted with AlCl3 gives BCl3 via halogen exchange. BCl3 is a trigonal planar molecule like the other boron trihalides, and has a bond length of 175pm. A degree of π-bonding has been proposed to explain the short B− Cl distance although there is some debate as to its extent. It does not dimerize, although NMR studies of mixtures of boron trihalides shows the presence of mixed halides. The absence of dimerisation contrasts with the t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutyl Vinyl Ether
In organic chemistry, butyl is a four- carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl Perchlorate
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl, although this term is barely heard. The acetyl group contains a methyl group () single-bonded to a carbonyl (). The carbonyl center of an acyl radical has one nonbonded electron with which it forms a chemical bond to the remainder ''R'' of the molecule. The acetyl moiety is a component of many organic compounds, including acetic acid, the neurotransmitter acetylcholine, acetyl-CoA, acetylcysteine, acetaminophen (also known as paracetamol), and acetylsalicylic acid (also known as aspirin). Acetylation In nature The introduction of an acetyl group into a molecule is called acetylation. In biological organisms, acetyl groups are commonly transferred from acetyl-CoA to other organic molecules. Acetyl-CoA is an intermediate both ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Salt
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylsulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula ( CH3)2. This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO has the unusual property that many individuals perceive a garlic-like taste in the mouth after DMSO makes contact with their skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Dimethyl sulfoxide is produced industrially from dimethyl sulfide, a by-product of the Kraft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylsulfide
Dimethyl sulfide (DMS) or methylthiomethane is an organosulfur compound with the formula (CH3)2S. Dimethyl sulfide is a flammable liquid that boils at and has a characteristic disagreeable odor. It is a component of the smell produced from cooking of certain vegetables, notably maize, cabbage, beetroot, and seafoods. It is also an indication of bacterial contamination in malt production and brewing. It is a breakdown product of dimethylsulfoniopropionate (DMSP), and is also produced by the bacterial metabolism of methanethiol. Occurrence and production DMS originates primarily from DMSP, a major secondary metabolite in some marine algae. DMS is the most abundant biological sulfur compound emitted to the atmosphere. Emission occurs over the oceans by phytoplankton. DMS is also produced naturally by bacterial transformation of dimethyl sulfoxide (DMSO) waste that is disposed of into sewers, where it can cause environmental odor problems. DMS is oxidized in the marine atmosphere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvent Polarity
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners ( toluene, turpentine); as nail polish removers and solvents of glue ( acetone, methyl acetate, ethyl acetate); in spot removers ( hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes ( ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-butyl Chloride
''tert''-Butyl chloride is the organochloride with the formula . It is a colorless, flammable liquid. It is sparingly soluble in water, with a tendency to undergo hydrolysis to the corresponding ''tert''-butyl alcohol. It is produced industrially as a precursor to other organic compounds.M. Rossberg et al. "Chlorinated Hydrocarbons" in ''Ullmann's Encyclopedia of Industrial Chemistry'' 2006, Wiley-VCH, Weinheim. Synthesis ''tert''-Butyl chloride is produced by the reaction of ''tert''-butyl alcohol with hydrogen chloride. In the laboratory, concentrated hydrochloric acid is used. The conversion entails a SN1 reaction as shown below.James F. Norris and Alanson W. Olmsted "''tert''-Butyl Chloride" Org. Synth. 1928, volume 8, pp. 50. The overall reaction, therefore, is: : Because ''tert''-butanol is a tertiary alcohol, the relative stability of the ''tert''-butyl carbocation in the step 2 allows the SN1, SN1 mechanism to be followed, whereas a primary alcohol would follow a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethylaluminium Chloride
Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although usually given the chemical formula (C2H5)2AlCl, it exists as a dimer, C2H5)2AlClsub>2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric. Structure Compounds of the empirical formula AlR2Cl (R = alkyl, aryl) exist as dimers with the formula (R2Al)2(μ-Cl)2. The aluminium adopts a tetrahedral geometry. Production Diethylaluminium chloride can be produced from ethylaluminium sesquichloride, (C2H5)3Al2Cl3, by reduction with sodium: :2 (C2H5)3Al2Cl3 + 3 Na → 3 (C2H5)2AlCl + Al + 3 NaCl It is also obtained from the reaction of triethylaluminium Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
s.png)