|
Lithium Molybdenum Purple Bronze
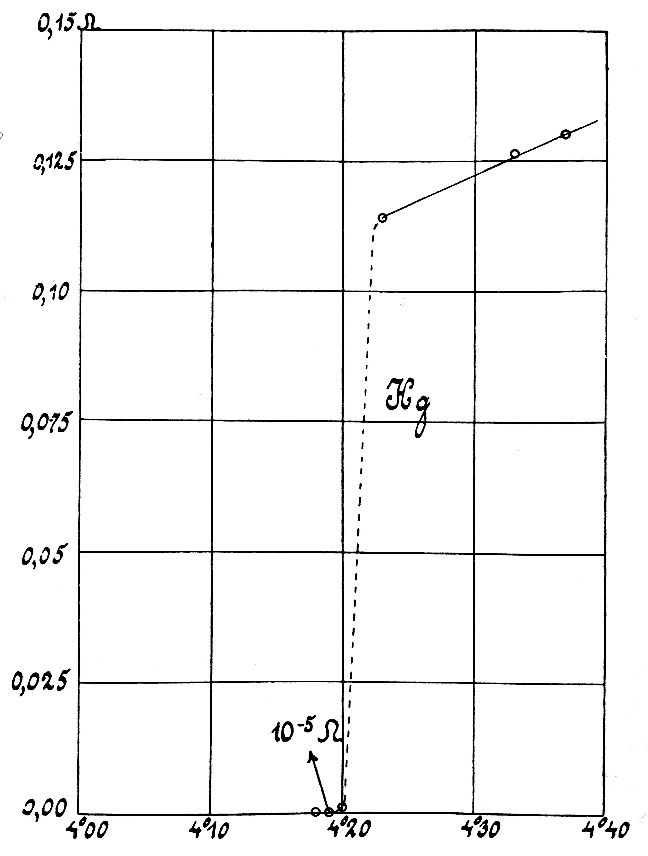
Lithium molybdenum purple bronze is a chemical compound with formula , that is, a mixed oxide of molybdenum and lithium. It can be obtained as flat crystals with a purple-red color and metallic sheen (hence the "purple bronze" name). This compound is one of several molybdenum bronzes with general formula where A is an alkali metal or thallium Tl. It stands out among them (and also among the sub-class of "purple" molybdenum bronzes) for its peculiar electrical properties, including a marked isotropic, anisotropy that makes it a "quasi-1D" conductor, and a metal-to-insulator transition as it is cooled below 30 kelvin, K. Preparation The compound was first obtained by Martha Greenblatt and others by a temperature gradient flux technique. In a typical preparation, a stoichometric melt of , and is maintained in a temperature gradient from 490 to 640 °C oven 15 cm in vacuum over several days. Excess reagents are dissolved with a hot potassium carbonate solution releasing met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gram
The gram (originally gramme; SI unit symbol g) is a Physical unit, unit of mass in the International System of Units (SI) equal to one one thousandth of a kilogram. Originally defined as of 1795 as "the absolute weight of a volume of pure water equal to Cube (algebra), the cube of the hundredth part of a metre [1 Cubic centimetre, cm3], and at Melting point of water, the temperature of Melting point, melting ice", the defining temperature (~0 °C) was later changed to 4 °C, the temperature of maximum density of water. However, by the late 19th century, there was an effort to make the Base unit (measurement), base unit the kilogram and the gram a derived unit. In 1960, the new International System of Units defined a ''gram'' as one one-thousandth of a kilogram (i.e., one gram is Scientific notation, 1×10−3 kg). The kilogram, 2019 redefinition of the SI base units, as of 2019, is defined by the International Bureau of Weights and Measures from the fixed numeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Tungsten Bronze
Sodium tungsten bronze is a form of insertion compound with the formula Na''x''WO3, where ''x'' is equal to or less than 1. So named because of its metallic lustre, its electrical properties range from semiconducting to metallic depending on the concentration of sodium ions present; it can also exhibit superconductivity. History Prepared in 1823 by the chemist Friedrich Wöhler, sodium tungsten bronze was the first alkali metal bronze to be discovered. Tungsten bronzes owe some of their properties to the relative stability of the tungsten(V) cation that is formed. A similar family of molybdenum bronzes may have been discovered in 1885 by Alfred Stavenhagen and E. Engels, but they are formed in a very narrow range of temperatures and were not reported again until the 1960s. Properties Sodium tungsten bronze, like other tungsten bronzes, is resistant to chemical reaction under both acidic and basic conditions. Colour is dependent upon the proportion of sodium in the compound, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetoresistance
Magnetoresistance is the tendency of a material (often ferromagnetic) to change the value of its electrical resistance in an externally-applied magnetic field. There are a variety of effects that can be called magnetoresistance. Some occur in bulk non-magnetic metals and semiconductors, such as geometrical magnetoresistance, Shubnikov–de Haas oscillations, or the common positive magnetoresistance in metals. Other effects occur in magnetic metals, such as negative magnetoresistance in ferromagnets or anisotropic magnetoresistance (AMR). Finally, in multicomponent or multilayer systems (e.g. magnetic tunnel junctions), giant magnetoresistance (GMR), tunnel magnetoresistance (TMR), colossal magnetoresistance (CMR), and extraordinary magnetoresistance (EMR) can be observed. The first magnetoresistive effect was discovered in 1856 by William Thomson, better known as Lord Kelvin, but he was unable to lower the electrical resistance of anything by more than 5%. Today, systems includi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to conduct heat. It is commonly denoted by k, \lambda, or \kappa. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials like Rockwool or Styrofoam. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla T is the temperature gradient. This is known as Fourier's Law for heat conduction. Although commonly expressed as a scalar, the most general form of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin–charge Separation
In condensed matter physics, spin–charge separation is an unusual behavior of electrons in some materials in which they 'split' into three independent particles, the spinon, the orbiton and the holon (or chargon). The electron can always be theoretically considered as a bound state of the three, with the spinon carrying the spin of the electron, the orbiton carrying the orbital degree of freedom and the chargon carrying the charge, but in certain conditions they can behave as independent quasiparticles. The theory of spin–charge separation originates with the work of Sin-Itiro Tomonaga who developed an approximate method for treating one-dimensional interacting quantum systems in 1950. This was then developed by Joaquin Mazdak Luttinger in 1963 with an exactly solvable model which demonstrated spin–charge separation. In 1981 F. Duncan M. Haldane generalized Luttinger's model to the Tomonaga–Luttinger liquid concept whereby the physics of Luttinger's model was shown the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Density Wave
A charge density wave (CDW) is an ordered quantum fluid of electrons in a linear chain compound or layered crystal. The electrons within a CDW form a standing wave pattern and sometimes collectively carry an electric current. The electrons in such a CDW, like those in a superconductor, can flow through a linear chain compound en masse, in a highly correlated fashion. Unlike a superconductor, however, the electric CDW current often flows in a jerky fashion, much like water dripping from a faucet due to its electrostatic properties. In a CDW, the combined effects of pinning (due to impurities) and electrostatic interactions (due to the net electric charges of any CDW kinks) likely play critical roles in the CDW current's jerky behavior, as discussed in sections 4 & 5 below. Most CDW's in metallic crystals form due to the wave-like nature of electrons – a manifestation of quantum mechanical wave-particle duality – causing the electronic charge density to become spatially modula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Resistivity And Conductivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). For example, if a solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is , then the resistivity of the material is . Electrical conductivity or specific conductance is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter ( sigma), but (kappa) (especially in electrical engineering) and (gamma) are sometimes used. The SI unit of electrical conductivity is siemens per metre (S/m). Resistivity and conductivity are intensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ohm Centimetre
Ohm (symbol Ω) is a unit of electrical resistance named after Georg Ohm. Ohm or OHM may also refer to: People * Georg Ohm (1789–1854), German physicist and namesake of the term ''ohm'' * Germán Ohm (born 1936), Mexican boxer * Jörg Ohm (born 1944), former East German football player * Martin Ohm (1792–1872), German mathematician * Rebecca Ohm, United States Air Force officer and fighter pilot * Rune Ohm (born 1980), Danish handball player * Thorsten Ohm, CEO of VDM Publishing * Pawat Chittsawangdee, Thai actor, nicknamed Ohm Places Germany * Ohm (river), right tributary of the Lahn near Cölbe * Zwester Ohm, left tributary of the Lahn near Fronhausen Outer space * 24750 Ohm, an outer main belt asteroid * Ohm (crater) on the Moon Science and technology * Acoustic ohm, a unit of measurement of acoustic impedance * Ohm's law, law that relates electrical resistance, current, and voltage * OHM (Observe. Hack. Make.), a 2013 outdoor hacker conference * OpenHistoricalMap, a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows electric current. Resistivity is commonly represented by the Greek letter (rho). The SI unit of electrical resistivity is the ohm-meter (Ω⋅m). For example, if a solid cube of material has sheet contacts on two opposite faces, and the resistance between these contacts is , then the resistivity of the material is . Electrical conductivity or specific conductance is the reciprocal of electrical resistivity. It represents a material's ability to conduct electric current. It is commonly signified by the Greek letter ( sigma), but (kappa) (especially in electrical engineering) and (gamma) are sometimes used. The SI unit of electrical conductivity is siemens per metre (S/m). Resistivity and conductivity are intensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)