|
Lithium Diphenylphosphide
Lithium diphenylphosphide contains lithium and the organophosphorus anion with the formula (C6H5)2PLi. It is an air-sensitive solid that is used in the preparation of diphenylphosphino compounds. As an ether complex, the lithium salt is dark red. Synthesis and reactions The lithium, sodium, and potassium salts are prepared by reduction of chlorodiphenylphosphine, triphenylphosphine, or tetraphenyldiphosphine with alkali metals (M): :(C6H5)2PCl + 2 M → (C6H5)2PM + MCl :(C6H5)3P + 2 M → (C6H5)2PM + MC6H5 :(C6H5)4P2 + 2 M → 2 (C6H5)2PM They can also be obtained by deprotonation of diphenylphosphine. With water, the salts convert to diphenylphosphine: :(C6H5)2PLi + H2O → (C6H5)2PH + LiOH With halocarbons, the salts react to give tertiary phosphines: :(C6H5)2PM + RX → (C6H5)2PR + MX When treated with metal halides, lithium diphenylphosphide gives transition metal phosphido complexes. Structure Although treated as salts, alkali diphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorodiphenylphosphine
Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is useful reagent for introducing the Ph2P group into molecules, which includes many ligands.Quin, L. D. ''A Guide to Organophosphorus Chemistry''; Wiley IEEE: New York, 2000; pp 44-69. Like other halophosphines, Ph2PCl is reactive with many nucleophiles such as water and easily oxidized even by air. Synthesis and reactions Chlorodiphenylphosphine is produced on a commercial scale from benzene and phosphorus trichloride (PCl3). Benzene reacts with phosphorus trichloride at extreme temperatures around 600 °C to give dichlorophenylphosphine (PhPCl2) and HCl. Redistribution of PhPCl2 in the gas phase at high temperatures results in chlorodiphenylphosphine. :2PhPCl2 → Ph2PCl + PCl3 Alternatively such compounds are prepared by redistribu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes slow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetraphenyldiphosphine
Tetraphenyldiphosphine is the organophosphorus compound with the formula Ph2sub>2, where Ph = phenyl (C6H5). It is a white, air-sensitive solid that dissolves in nonpolar solvents. It is a centrosymmetric molecule with a P-P bond of 2.2592 Å. Tetraphenyldiphosphine is produced by reductive coupling of chlorodiphenylphosphine Chlorodiphenylphosphine is an organophosphorus compound with the formula (C6H5)2PCl, abbreviated Ph2PCl. It is a colourless oily liquid with a pungent odor that is often described as being garlic-like and detectable even in the ppb range. It is u ...: :2 Ph2PCl + 2 Na → Ph2P-PPh2 + 2 NaCl The compound is used as a source of the Ph2P− group.{{cite journal, title=Zur Kenntnis der Organophosphorverbindungen, III. Umsetzungen mit Diphenylphosphin‐natrium , first1=Wilhelm , last1=Kuchen, first2=Hans, last2=Buchwald, journal=Chem. Ber., volume=92, pages=227–231 , year=1959, doi=10.1002/cber.19590920126 :Ph2P-PPh2 + 2 Na → + 2 NaPPh2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylphosphine
Diphenylphosphine, also known as diphenylphosphane, is an organophosphorus compound with the formula (C6H5)2PH. This foul-smelling, colorless liquid is easily oxidized in air. It is a precursor to organophosphorus ligands for use as catalysts. Synthesis Diphenylphosphine can be prepared from triphenylphosphine by reduction to lithium diphenylphosphide, which can be protonated to give the title compound: :PPh3 + 2 Li → LiPPh2 + LiPh :LiPPh2 + H2O → Ph2PH + LiOH Uses and reactions In the laboratory, diphenylphosphine is a common intermediate. It can be deprotonated to give diphenylphosphide derivatives: :Ph2PH + nBuLi → Ph2PLi + nBuH The preparation of phosphine ligands, Wittig-Horner reagents, and phosphonium salts are commonly accomplished by alkylating diphenylphosphine. The hydrogen atom connected to phosphorus undergoes Michael-like addition to activated alkenes, providing products with which to produce phosphine ligands such as 1,2-bis(diphenylphosphino)ethane 1,2-Bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halocarbon
Halocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides. Many synthetic organic compounds such as plastic polymers, and a few natural ones, contain halogen atoms; they are known as ''halogenated'' compounds or ''organohalogens''. Organochlorides are the most common industrially used organohalides, although the other organohalides are used commonly in organic synthesis. Except for extremely rare cases, organohalides are not produced biologically, but many pharmaceuticals are organohalides. Notably, many pharmaceuticals such as Prozac have trifluoromethyl groups. For information on inorganic halide chemistry, see halide. Chemical families Halocarbons are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition Metal Phosphido Complexes
A transition metal phosphido complex is a coordination complex containing a phosphido ligand (R2P, where R = H, organic substituent). With two lone pairs on phosphorus, the phosphido anion (R2P−) is comparable to an amido anion (R2N−), except that the M-P distances are longer and the phosphorus atom is more sterically accessible. For these reasons, phosphido is often a bridging ligand. The -PH2 ion or ligand is also called phosphanide or phosphido ligand. Synthesis Phosphido ligands are often installed by salt metathesis reactions. Sources of R2P+ and R2P− are provided by phosphorus halides and alkali metal phosphides respectively. Illustrative of the use of R2PCl-like reagents is the synthesis of a diiron diphosphide: :Na2Fe2(CO)8 + 2 Ph2PCl → Fe2(PPh2)2(CO)6 + 2 NaCl + 2 CO The alternative salt metathesis route involves the reaction of alkali metal diorganophosphides with metal halides. A typical phosphide reagent is lithium diphenylphosphide. Alkali met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
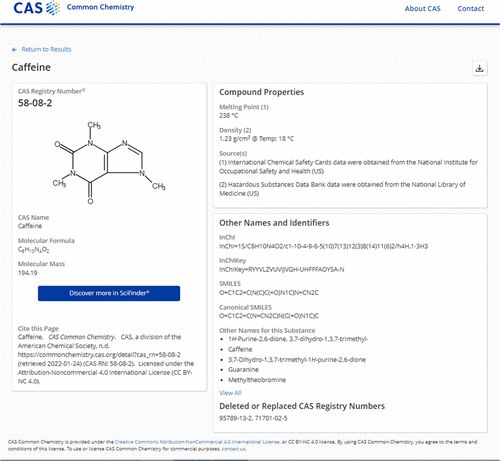
CAS Registry Number
A CAS Registry Number (also referred to as CAS RN or informally CAS Number) is a unique identification number assigned by the Chemical Abstracts Service (CAS), US to every chemical substance described in the open scientific literature. It includes all substances described from 1957 through the present, plus some substances from as far back as the early 1800s. It is a chemical database that includes organic and inorganic compounds, minerals, isotopes, alloys, mixtures, and nonstructurable materials (UVCBs, substances of unknown or variable composition, complex reaction products, or biological origin). CAS RNs are generally serial numbers (with a check digit), so they do not contain any information about the structures themselves the way SMILES and InChI strings do. The registry maintained by CAS is an authoritative collection of disclosed chemical substance information. It identifies more than 182 million unique organic and inorganic substances and 68 million protein and DNA seq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Salts
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes found i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Compounds
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and deve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Compounds
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |