|
List Of Alcohols
This list is ordered by the number of carbon atoms in an alcohol. C1 * Methanol C2 * Ethanol C3 * 1-Propanol * Allyl alcohol * Isopropyl alcohol C4 * n-Butanol * Isobutanol * sec-Butanol * tert-Butyl alcohol C5 * 1-Pentanol * Isoamyl alcohol * 2-Methyl-1-butanol * Neopentyl alcohol * 2-Pentanol * 3-Methyl-2-butanol * 3-Pentanol * tert-Amyl alcohol C6 {{See also, Hexanol * 1-Hexanol * 2-Hexanol * 3-Hexanol * 2-Methyl-1-pentanol * 3-Methyl-1-pentanol * 4-Methyl-1-pentanol * 2-Methyl-2-pentanol * 3-Methyl-2-pentanol * 4-Methyl-2-pentanol * 2-Methyl-3-pentanol * 3-Methyl-3-pentanol * 2,2-Dimethyl-1-butanol * 2,3-Dimethyl-1-butanol * 3,3-Dimethyl-1-butanol Alkanols, Chemistry-related lists, Alcohols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy hu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Hexanol
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry. Preparation Hexanol is produced industrially by the oligomerization of ethylene using triethylaluminium followed by oxidation of the alkylaluminium products.. An idealized synthesis is shown: :Al(C2H5)3 + 6C2H4 → Al(C6H13)3 :Al(C6H13)3 + O2 + 3H2O → 3HOC6H13 + Al(OH)3 The process generates a range of oligomers that are separated by distillation. Alternative methods Another method of preparation entails hydroformylation of 1-pentene followed by hydrogenation of the resulting aldehydes. This method is practiced in indu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2-Dimethyl-1-butanol
2,2-Dimethyl-1-butanol is an organic chemical compound; it is one of the isomeric hexanols. Its main use is as a solvent A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for .... References Hexanols Primary alcohols {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
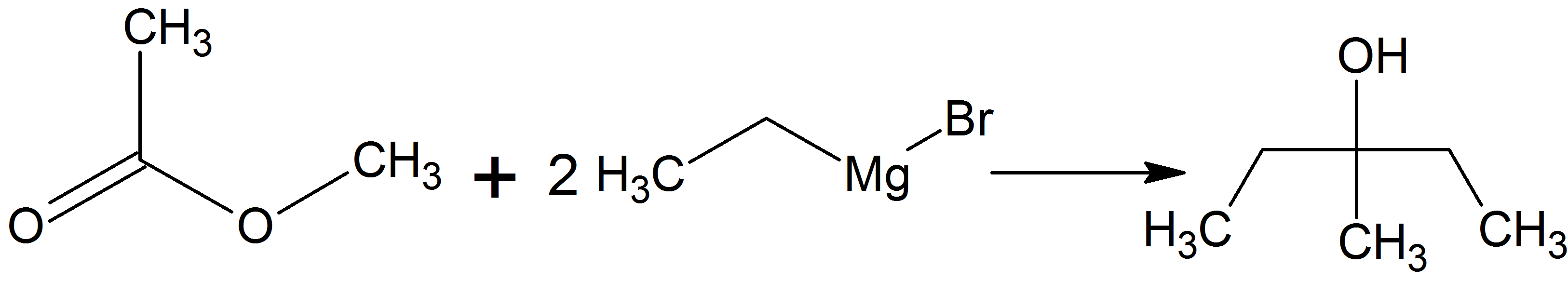
3-Methyl-3-pentanol
3-Methyl-3-pentanol (IUPAC name: 3-methylpentan-3-ol) is an organic chemical compound and a tertiary hexanol. It is used in the synthesis of the tranquilizer emylcamate, and has similar sedative and anticonvulsant actions itself. Synthesis It can be prepared by reacting ethylmagnesium bromide with methyl acetate in the so-called Grignard reaction using dried diethyl ether or tetrahydrofuran as solvent. It can be prepared also by reacting ethylmagnesium bromide with butanone Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in ... in the same conditions already mentioned. References Tertiary alcohols Hexanols {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Methyl-2-pentanol
4-Methyl-2-pentanol (IUPAC name: 4-methylpentan-2-ol) or methyl isobutyl carbinol (MIBC) is an organic chemical compound used primarily as a frother in mineral flotation and in the production of lubricant oil additives such as Zinc dithiophosphate. It is also used as a solvent, in organic synthesis, and in the manufacture of brake fluid and as a precursor to some plasticizer A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture. Plasticiz ...s. It is an acetone derivative in liquid state, with limited solubility in water but generally miscible with most organic solvents. References Hexanols {{mining-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Methyl-2-pentanol
2-Methyl-2-pentanol (IUPAC name: 2-methylpentan-2-ol) is an organic chemical compound. It can be added to a gas chromatograph to help distinguish between branched compounds, especially alcohols. Its presence in urine can be used to test for exposure to 2-methylpentane. As with many other short-chain alcohols, 2-methyl-2-pentanol can produce intoxication and sedative effects similar to those of ethanol Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ..., though it is more irritating to mucous membranes and generally more toxic to the body. See also * 2-Methyl-2-butanol * 3-Methyl-3-pentanol References Hexanols Tertiary alcohols {{alcohol-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |