|
List Of Enzymes
This article lists enzymes by their classification in the International Union of Biochemistry and Molecular Biology's Enzyme Commission (EC) numbering system. * List of EC numbers (EC 5) * List of EC numbers (EC 6) :Oxidoreductases (EC 1) (Oxidoreductase) *Dehydrogenase * Luciferase *DMSO reductase :EC 1.1 (act on the CH-OH group of donors) * :EC 1.1.1 (with NAD+ or NADP+ as acceptor) ** Alcohol dehydrogenase (NAD) ** Alcohol dehydrogenase (NADP) **Homoserine dehydrogenase ** Aminopropanol oxidoreductase **Diacetyl reductase **Glycerol dehydrogenase **Propanediol-phosphate dehydrogenase ** glycerol-3-phosphate dehydrogenase (NAD+) ** D-xylulose reductase **L-xylulose reductase **Lactate dehydrogenase **Malate dehydrogenase **Isocitrate dehydrogenase ** HMG-CoA reductase * :EC 1.1.2 (with a cytochrome as acceptor) * :EC 1.1.3 (with oxygen as acceptor) **Glucose oxidase **L-gulonolactone oxidase **Thiamine oxidase **Xanthine oxidase * :EC 1.1.4 (with a disul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propanediol-phosphate Dehydrogenase
In enzymology, a propanediol-phosphate dehydrogenase () is an enzyme that catalyzes the chemical reaction :propane-1,2-diol 1-phosphate + NAD+ \rightleftharpoons hydroxyacetone phosphate + NADH + H+ Thus, the two substrates of this enzyme are propane-1,2-diol 1-phosphate and NAD+, whereas its 3 products are hydroxyacetone phosphate, NADH, and H+. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with NAD+ or NADP+ as acceptor. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial ... of this enzyme class is propane-1,2-diol-1-phosphate:NAD+ oxidoreductase. Other names in common use include PDP dehydrogenase, 1,2-propanediol-1-phosphate:NAD+ oxidoreductase, and propanediol phosphate dehydrogenase. Refere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
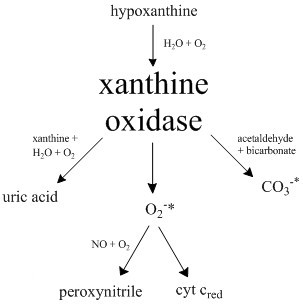
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiamine Oxidase
In enzymology, a thiamine oxidase () is an enzyme that catalyzes the chemical reaction ::thiamine + 2 O2 + H2O \rightleftharpoons thiamine acetic acid + 2 H2O2 The 3 substrates of this enzyme are thiamine, O2, and H2O, whereas its two products are thiamine acetic acid and H2O2. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with oxygen as acceptor. The systematic name of this enzyme class is thiamine:oxygen 5-oxidoreductase. Other names in common use include thiamin dehydrogenase, thiamine dehydrogenase, and thiamin:oxygen 5-oxidoreductase. This enzyme participates in thiamine metabolism. It employs one cofactor, FAD A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period. Fads are objects or behaviors that achieve short- .... References * * * EC 1.1.3 Flav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-gulonolactone Oxidase
L-Gulonolactone oxidase ( ECbr>1.1.3.8 is an enzyme that produces vitamin C, but is non-functional in Haplorrhini (including humans), in some bats, and in guinea pigs. It catalyzes the reaction of L-gulono-1,4-lactone with oxygen to form L-xylo-hex-3-gulonolactone (2-keto-gulono-γ-lactone) and hydrogen peroxide. It uses FAD as a cofactor. The L-xylo-hex-3-gulonolactone then converts to hexuronic acid ( ascorbic acid) spontaneously, without enzymatic action. Gulonolactone oxidase deficiency The non-functional gulonolactone oxidase pseudogene (''GULOP'') was mapped to human chromosome 8p21, which corresponds to an evolutionarily conserved segment on either porcine chromosome 4 (SSC4) or 14 (SSC14). GULO produces the precursor to ascorbic acid, which spontaneously converts to the vitamin itself. The loss of activity of the gene encoding L-gulonolactone oxidase (GULO) has occurred separately in the history of several species. GULO activity has been lost in some species of bats ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose Oxidase
The glucose oxidase enzyme (GOx or GOD) also known as notatin (EC number 1.1.3.4) is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and insects and displays antibacterial activity when oxygen and glucose are present. Glucose oxidase is widely used for the determination of free glucose in body fluids (medical testing), in vegetal raw material, and in the food industry. It also has many applications in biotechnologies, typically enzyme assays for biochemistry including biosensors in nanotechnologies. It was first isolated by Detlev Müller in 1928 from ''Aspergillus niger''. Function Several species of fungi and insects synthesize glucose oxidase, which produces hydrogen peroxide, which kills bacteria. Notatin, extracted from antibacterial cultures of ''Penicillium notatum'', was originally named Penicillin A, but was renamed to avoid confusion with penicillin. Notati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d. Cytochrome function is linked to the reversible redox change from ferrous (Fe(II)) to the ferric (Fe(III)) oxidation state of the iron found in the heme core. In addition to the classification by the IUBMB into four cytochrome classes, several additional classifications such as cytochrome o and cytochrome P450 can be found in biochemical literature. History Cytochromes were initially described in 1884 by Charles Alexander MacMunn as respiratory pigments (myohematin or histohematin). In the 1920s, Keilin rediscovered these respiratory pigments and named them the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HMG-CoA Reductase
HMG-CoA reductase (3-hydroxy-3-methyl-glutaryl-coenzyme A reductase, official symbol HMGCR) is the rate-controlling enzyme (NADH-dependent, ; NADPH-dependent, ) of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. HMGCR catalyzes the conversion of HMG-CoA to mevalonic acid, a necessary step in the biosynthesis of cholesterol. Normally in mammalian cells this enzyme is competitively suppressed so that its effect is controlled. This enzyme is the target of the widely available cholesterol-lowering drugs known collectively as the statins, which help treat dyslipidemia. HMG-CoA reductase is anchored in the membrane of the endoplasmic reticulum, and was long regarded as having seven transmembrane domains, with the active site located in a long carboxyl terminal domain in the cytosol. More recent evidence shows it to contain eight transmembrane domains. In humans, the gene for HMG-CoA reductase (NADPH) is located on the long arm of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocitrate Dehydrogenase
Isocitrate dehydrogenase (IDH) () and () is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome. Isozymes The following is a list of human isocitrate dehydrogenase isozymes: NADP+ dependent Each NADP+-dependent isozyme functions as a homodimer: See also * Isocitrate/isopropylmalate dehydrogenase family NAD+ depe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malate Dehydrogenase
Malate dehydrogenase () (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate dehydrogenases, which have other EC numbers and catalyze other reactions oxidizing malate, have qualified names like malate dehydrogenase (NADP+). Isozymes Several isozymes of malate dehydrogenase exist. There are two main isoforms in eukaryotic cells. One is found in the mitochondrial matrix, participating as a key enzyme in the citric acid cycle that catalyzes the oxidation of malate. The other is found in the cytoplasm, assisting the malate-aspartate shuttle with exchanging reducing equivalents so that malate can pass through the mitochondrial membrane to be transformed into oxaloacetate for further cellular processes. Humans and most other mammals express the following two malate dehydrogenases: Protein families The m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactate Dehydrogenase
Lactate dehydrogenase (LDH or LD) is an enzyme found in nearly all living cells. LDH catalyzes the conversion of lactate to pyruvate and back, as it converts NAD+ to NADH and back. A dehydrogenase is an enzyme that transfers a hydride from one molecule to another. LDH exists in four distinct enzyme classes. This article is specifically about the NAD(P)-dependent L-lactate dehydrogenase. Other LDHs act on D-lactate and/or are dependent on cytochrome c: D-lactate dehydrogenase (cytochrome) and L-lactate dehydrogenase (cytochrome). LDH is expressed extensively in body tissues, such as blood cells and heart muscle. Because it is released during tissue damage, it is a marker of common injuries and disease such as heart failure. Reaction Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+. It converts pyruvate, the final product of glycolysis, to lactate when oxygen is absent or in short supp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |