|
Liquid Organic Hydrogen Carriers
Liquid organic hydrogen carriers (LOHC) are organic compounds that can absorb and release hydrogen through chemical reactions. LOHCs can therefore be used as storage media for hydrogen. In principle, every unsaturated compound (organic molecules with C-C double or triple bonds) can take up hydrogen during hydrogenation. The sequence of endothermal dehydrogenation followed by hydrogen purification is considered as the main drawback which limits the overall efficiency of the storage cycle.G. Sievi, D. Geburtig, T. Skeledzic, A. Bösmann, P. Preuster, O. Brummel, ... & J. Libuda (2019). ''Towards an efficient liquid organic hydrogen carrier fuel cell concept''. In: ''Energy & Environmental Science'', 12(7), 2305-2314. In 2020, Japan built up the world's first international hydrogen supply chain between Brunei and Kawasaki City utilizing toluene-based LOHC technology. Hyundai Motor invests in the development for stationary and on-board LOHC-systems. Principle of LOHC-based hydrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reaction
In thermochemistry, an exothermic reaction is a "reaction for which the overall standard enthalpy change Δ''H''⚬ is negative." Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall standard Gibbs energy change Δ''G''⚬ is negative." A strongly exothermic reaction will usually also be exergonic because Δ''H''⚬ makes a major contribution to Δ''G''⚬. Most of the spectacular chemical reactions that are demonstrated in classrooms are exothermic and exergonic. The opposite is an endothermic reaction, which usually takes up heat and is driven by an entropy increase in the system. Examples Examples are numerous: combustion, the thermite reaction, combining strong acids and bases, polymerizations. As an example in everyday life, hand warmers make use of the oxidation of iron to achieve an exothermic reaction: :4Fe + 3O2 → 2Fe2O3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylcyclohexane
Methylcyclohexane (cyclohexylmethane) is an organic compound with the molecular formula is CH3C6H11. Classified as saturated hydrocarbon, it is a colourless liquid with a faint odor. Methylcyclohexane is used as a solvent. It is mainly converted in naphtha reformers to toluene.M. Larry Campbell. "Cyclohexane" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2012. Methylcyclohexane is also used in some correction fluids (such as White-Out) as a solvent. Production and use It can be also produced by hydrogenation of toluene: :CH3C6H5 + 3 H2 → CH3C6H11 Methylcyclohexane, as a component of a mixture, is usually dehydrogenated to toluene, which increases the octane rating of gasoline. : It is also one of a host substances in jet fuel surrogate blends, e.g., for Jet A fuel. Solvent Methylcyclohexane is used as an organic solvent, with properties similar to related saturated hydrocarbons such as heptane. It is also a solvent in many types of corr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PEMFC
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges (50 to 100 °C) and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle. Science PEMFCs are built out of membrane electrode assemblies (MEA) which include the electrodes, electrolyte, catalyst, and gas diffusion layers. An ink of catalyst, carbon, and electrode are sprayed or painted onto the solid electrolyte and carbon paper is hot pressed on either side to protect the inside of the cell and also act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-propanol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Alcohol
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour. Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
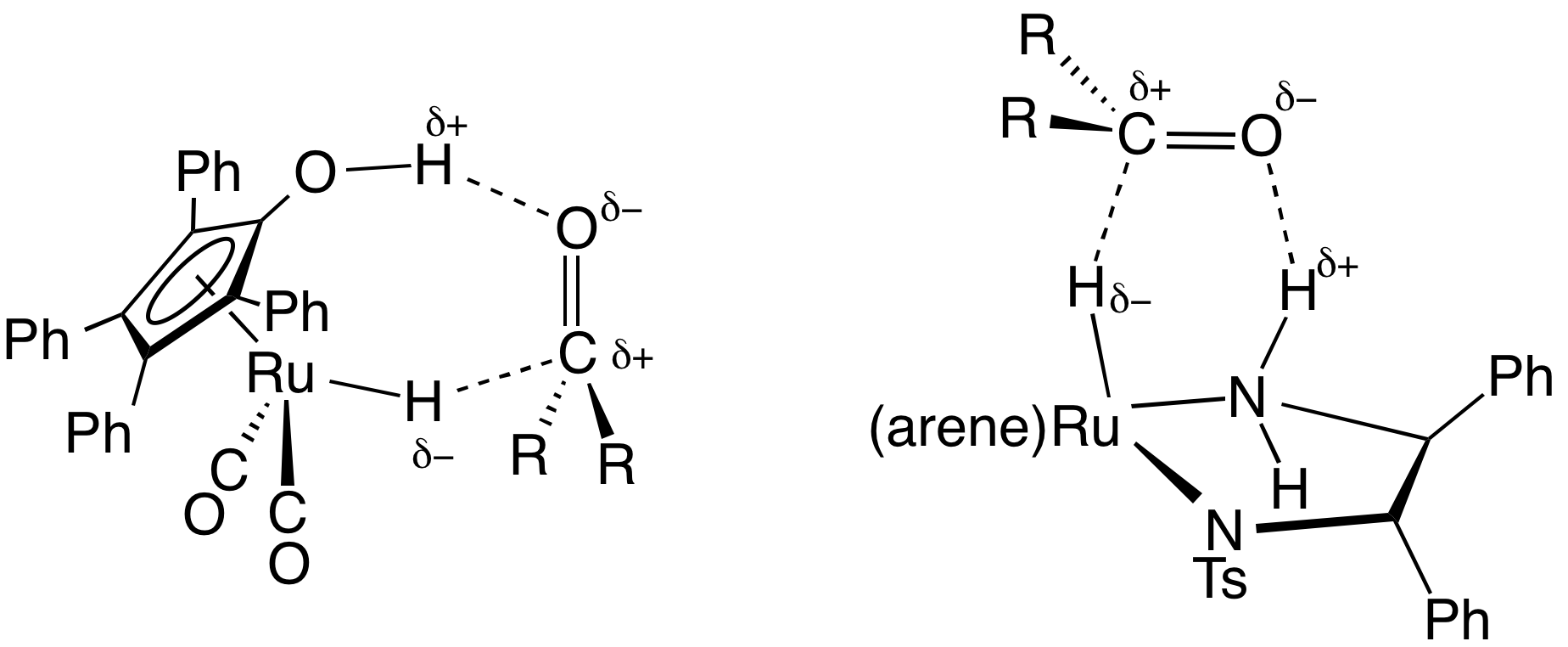
Transfer Hydrogenation
In chemistry, transfer hydrogenation is a chemical reaction involving the addition of hydrogen to a compound from a source other than molecular . It is applied in laboratory and industrial organic synthesis to saturate organic compounds and reduce ketones to alcohols, and imines to amines. It avoids the need for high-pressure molecular used in conventional hydrogenation. Transfer hydrogenation usually occurs at mild temperature and pressure conditions using organic or organometallic catalysts, many of which are chiral, allowing efficient asymmetric synthesis. It uses hydrogen donor compounds such as formic acid, isopropanol or dihydroanthracene, dehydrogenating them to , acetone, or anthracene respectively. Often, the donor molecules also function as solvents for the reaction. A large scale application of transfer hydrogenation is coal liquefaction using "donor solvents" such as tetralin. Organometallic catalysts In the area of organic synthesis, a useful family of hydrogen-trans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wolfgang Arlt
Wolfgang Arlt is a German thermodynamicist. Until his retirement in 2018, he was professor at the TU Berlin and since 2004 at the Friedrich-Alexander-Universität Erlangen-Nürnberg. Life After studying chemistry with a focus on physical chemistry at the University of Dortmund, he became a research assistant for Ulfert Onken at the same university in 1976 in the field of chemical engineering. During his doctorate, he helped set up the Dortmund Data Bank. After completing his doctorate as Dr.-Ing. In 1981 he moved to Bayer, where he worked on thermal separation processes. In 1987 he switched to plastics research in-house and worked in a leading position in setting up a production facility for a thermoplastic in Antwerp . After completing this work, he returned to the process engineering department in Leverkusen. In 1992 he accepted a position as professor for thermodynamics and thermal process engineering at Technische Universität Berlin . During this time he developed, among ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Wasserscheid
Peter Wasserscheid (born 23 October 1970, in Würzburg) is a German chemist and professor for chemical reaction engineering at the University of Erlangen-Nuremberg. Together with Matthias Beller he won the Gottfried Wilhelm Leibniz Prize in 2006. Personal background Wasserscheid studied chemistry at the RWTH Aachen from 1991 to 1995 before he did his doctorate in the work group of Professor Wilhelm Keim. After postdoctoral research at BP in Great Britain he habilitated at the RWTH Aachen. Since October 2003, Wasserscheid holds the chair of Chemical engineering at the University of Erlangen-Nuremberg. Wasserscheid is also a founder member of the company Solvent Innovation GmbH and Scientific Supervisor in this company since 2001. Wasserscheid is married and has 3 children. Work The focus of Wasserscheids work is ionic liquids where he is a pioneer, particularly in the region of developing halogenfree ionic liquids. Awards *1996: Friedrich-Wilhelm-Preis of the RWTH Aachen for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |