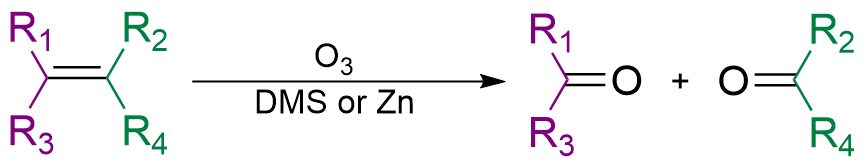|
Lemieux–Johnson Oxidation
The Lemieux–Johnson or Malaprade–Lemieux–Johnson oxidation is a chemical reaction in which an olefin undergoes oxidative cleavage to form two aldehyde or ketone units. The reaction is named after its inventors, Raymond Urgel Lemieux and William Summer Johnson, who published it in 1956. The reaction proceeds in a two step manner, beginning with dihydroxylation of the alkene by osmium tetroxide, followed by a Malaprade reaction to cleave the diol using periodate. Excess periodate is used to regenerate the osmium tetroxide, allowing it to be used in catalytic amounts. The Lemieux–Johnson reaction ceases at the aldehyde stage of oxidation and therefore produces the same results as ozonolysis. : The classical Lemieux–Johnson oxidation often generates many side products, resulting in low reaction yields; however the addition of non-nucleophilic bases, such as 2,6-lutidine, can improve on this. OsO4 may be replaced with a number of other Osmium compounds. Periodate may also be re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Oxidation Reactions
Organic may refer to: * Organic, of or relating to an organism, a living entity * Organic, of or relating to an anatomical organ Chemistry * Organic matter, matter that has come from a once-living organism, is capable of decay or is the product of decay, or is composed of organic compounds * Organic compound, a compound that contains carbon ** Organic chemistry, chemistry involving organic compounds Farming, certification and products * Organic farming, agriculture conducted according to certain standards, especially the use of stated methods of fertilization and pest control * Organic certification, accreditation process for producers of organically-farmed products * Organic horticulture, the science and art of growing fruits, vegetables, flowers, or ornamental plants by following the essential principles of organic agriculture * Organic products, "organics": ** Organic food, food produced from organic farming methods and often certified organic according to organic farming stan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl () group while azo compounds form nitrosamines (). The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions. Ozonolysis of alkenes Alkenes can be oxidized with ozone to form alcohols, aldehydes or ketones, or carboxylic acids. In a typical procedure, ozone is bubbled through a solution of the alkene in methanol at −78 °C until the solution takes on a characteristic blue color, which is due to unreacted ozone. This indicates complete consumption of the alkene. Alternatively, various other chemicals can be used as indicators of this endpoint by detecting the presence of ozone. If ozonolysis is performed by bubbling a stream of ozone-enriched oxygen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sharpless Asymmetric Dihydroxylation
Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alkenes of virtually every substitution, often high enantioselectivities are realized, with the chiral outcome controlled by the choice of dihydroquinidine (DHQD) vs dihydroquinine (DHQ) as the ligand. Asymmetric dihydroxylation reactions are also highly site selective, providing products derived from reaction of the most electron-rich double bond in the substrate. It is common practice to perform this reaction using a catalytic amount of osmium tetroxide, which after reaction is regenerated with reoxidants such as potassium ferricyanide or ''N''-methylmorpholine ''N''-oxide. This dramatically reduces the amount of the highly toxic and very expensive osmium tetroxide needed. These four reagents are commercially available premixed (" AD- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Milas Hydroxylation
The Milas hydroxylation is an organic reaction converting an alkene to a vicinal diol, and was developed by Nicholas A. Milas in the 1930s. The cis-diol is formed by reaction of alkenes with hydrogen peroxide and either ultraviolet light or a catalytic osmium tetroxide, vanadium pentoxide, or chromium trioxide. The reaction has been superseded in synthetic chemistry by the Upjohn dihydroxylation and later by the Sharpless asymmetric dihydroxylation Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence of a chiral quinine ligand to form a vicinal diol. The reaction has been applied to alk .... Mechanism The proposed mechanism for the Milas hydroxylation involves the initial combination of hydrogen peroxide and the osmium tetroxide catalyst to form an intermediate, which then adds to the alkene, followed by a cleavage that forms the product and regenerates the OsO4. Li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Upjohn Dihydroxylation
The Upjohn dihydroxylation is an organic reaction which converts an alkene to a ''cis'' vicinal diol. It was developed by V. VanRheenen, R. C. Kelly and D. Y. Cha of the Upjohn Company in 1976. It is a catalytic system using ''N''-methylmorpholine ''N''-oxide (NMO) as stoichiometric re-oxidant for the osmium tetroxide. It is superior to previous catalytic methods. Prior to this method, use of stoichiometric amounts of the toxic and expensive reagent osmium tetroxide was often necessary. The Upjohn dihydroxylation is still often used for the formation of ''cis''-vicinal diols; however, it can be slow and is prone to ketone byproduct formation. One of the peculiarities of the dihydroxylation of olefins is that the standard "racemic" method (the Upjohn dihydroxylation) is slower and often lower yielding than the asymmetric method (the Sharpless asymmetric dihydroxylation). Improvements to Upjohn dihydroxylation In response to these problems, Stuart Warren and co-worke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− centre is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Periodate
Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium ''meta''periodate (formula NaIO4) and sodium ''ortho''periodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents. Preparation Classically, periodate was most commonly produced in the form of sodium hydrogen periodate (). This is commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide. Or, similarly, from iodides by oxidation with bromine and sodium hydroxide: :\overset + Cl2 + 4 NaOH -> Na3H2IO6 + 2NaCl + H2O :NaI + 4 Br2 + 10 NaOH -> Na3H2IO6 + 8 NaBr + 4 H2O Modern industrial scale production involves the electrochemical oxidation of iodates, on a lead dioxide () anode, with the following standard electrode potential: :H5IO6 + H+ + 2e- -> IO3- + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent. It is the potassium salt of peroxymonosulfuric acid. Usually potassium peroxymonosulfate refers to the triple salt known as oxone. The standard electrode potential for potassium peroxymonosulfate is +1.81 V with a half reaction generating the hydrogen sulfate (): ::HSO5− + 2 H+ + 2 e− → HSO4− + H2O Oxone Potassium peroxymonosulfate per se is a relatively obscure salt, but its derivative called oxone is of commercial value. Oxone refers to the triple salt 2KHSO5·KHSO4·K2SO4. Oxone has a longer shelflife than does potassium peroxymonosulfate. A white, water-soluble solid, oxone loses <1% of its oxidizing power per month. Production Oxone is produced from peroxysulfuric acid, which is generated in situ by combining and |
Lemieux–Johnson Oxidation
The Lemieux–Johnson or Malaprade–Lemieux–Johnson oxidation is a chemical reaction in which an olefin undergoes oxidative cleavage to form two aldehyde or ketone units. The reaction is named after its inventors, Raymond Urgel Lemieux and William Summer Johnson, who published it in 1956. The reaction proceeds in a two step manner, beginning with dihydroxylation of the alkene by osmium tetroxide, followed by a Malaprade reaction to cleave the diol using periodate. Excess periodate is used to regenerate the osmium tetroxide, allowing it to be used in catalytic amounts. The Lemieux–Johnson reaction ceases at the aldehyde stage of oxidation and therefore produces the same results as ozonolysis. : The classical Lemieux–Johnson oxidation often generates many side products, resulting in low reaction yields; however the addition of non-nucleophilic bases, such as 2,6-lutidine, can improve on this. OsO4 may be replaced with a number of other Osmium compounds. Periodate may also be re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |