|
Lejía Lake
Laguna Lejía is a salt lake located in the Altiplano of the Antofagasta Region of northern Chile. The landscape of the area is dominated by the volcanoes Chiliques, Lascar, Aguas Calientes and Acamarachi. It is shallow and has no outlet, covering a surface area of about in the present-day. During glacial times, the lake was considerably larger owing to decreased evaporation and increased precipitation rates. It is populated by flamingos and a number of microorganisms. Geography and geology Lejía Lake lies in the Puna de Atacama of Chile, close to the border with Argentina. The city of San Pedro de Atacama lies northwest of Lejía Lake. The lake basin is surrounded by volcanoes, such as Aguas Calientes, Lascar, Tumisa, Lejía, Chiliques and Cordon de Puntas Negras, and smaller centres like Cerro Overo and La Albòndiga. The lake is endorheic and has a large catchment, and a lava flow forms its southern shore. Farther south lie two other lakes, Laguna Miscanti and Laguna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antofagasta Region
The Antofagasta Region ( es, Región de Antofagasta, ) is one of Chile's sixteen first-order administrative divisions. The second-largest region of Chile in area, it comprises three provinces, Antofagasta, El Loa and Tocopilla. It is bordered to the north by Tarapacá, by Atacama to the south, and to the east by Bolivia and Argentina. The region's capital is the port city of Antofagasta; another one of its important cities is Calama. The region's main economic activity is copper mining in its giant inland porphyry copper systems. Antofagasta's climate is extremely arid, albeit somewhat milder near the coast. Nearly all of the region is devoid of vegetation, except close to the Loa River and at oases such as San Pedro de Atacama. Much of the inland is covered by salt flats, tephra and lava flows, and the coast exhibits prominent cliffs. The region was sparsely populated by indigenous Changos and Atacameños until massive Chilean immigration in conjunction with a saltpeter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catchment
A drainage basin is an area of land where all flowing surface water converges to a single point, such as a river mouth, or flows into another body of water, such as a lake or ocean. A basin is separated from adjacent basins by a perimeter, the ''drainage divide'', made up of a succession of elevated features, such as ridges and hills. A basin may consist of smaller basins that merge at river confluences, forming a hierarchical pattern. Other terms for a drainage basin are catchment area, catchment basin, drainage area, river basin, water basin, and impluvium. In North America, they are commonly called a watershed, though in other English-speaking places, "watershed" is used only in its original sense, that of a drainage divide. In a closed drainage basin, or endorheic basin, the water converges to a single point inside the basin, known as a sink, which may be a permanent lake, a dry lake, or a point where surface water is lost underground. Drainage basins are similar but no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an Insulator (electricity), electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many are prepared from that acid. Spelling "Sulfate" is the spelling recommended by IUPAC, but "sulphate" was traditionally used in British English. Structure The sulfate anion consists of a central sulfur atom surrounded by four equivalent oxygen atoms in a tetrahedral arrangement. The symmetry is the same as that of methane. The sulfur atom is in the +6 oxidation state while the four oxygen atoms are each in the −2 state. The sulfate ion carries an overall charge of −2 and it is the conjugate base of the bisulfate (or hydrogensulfate) ion, , which is in turn the conjugate base of , sulfuric acid. Organic sulfate esters, such as dimethyl sulfate, are covalent compounds and esters of sulfuric acid. The tetrahedral molecular geometry of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
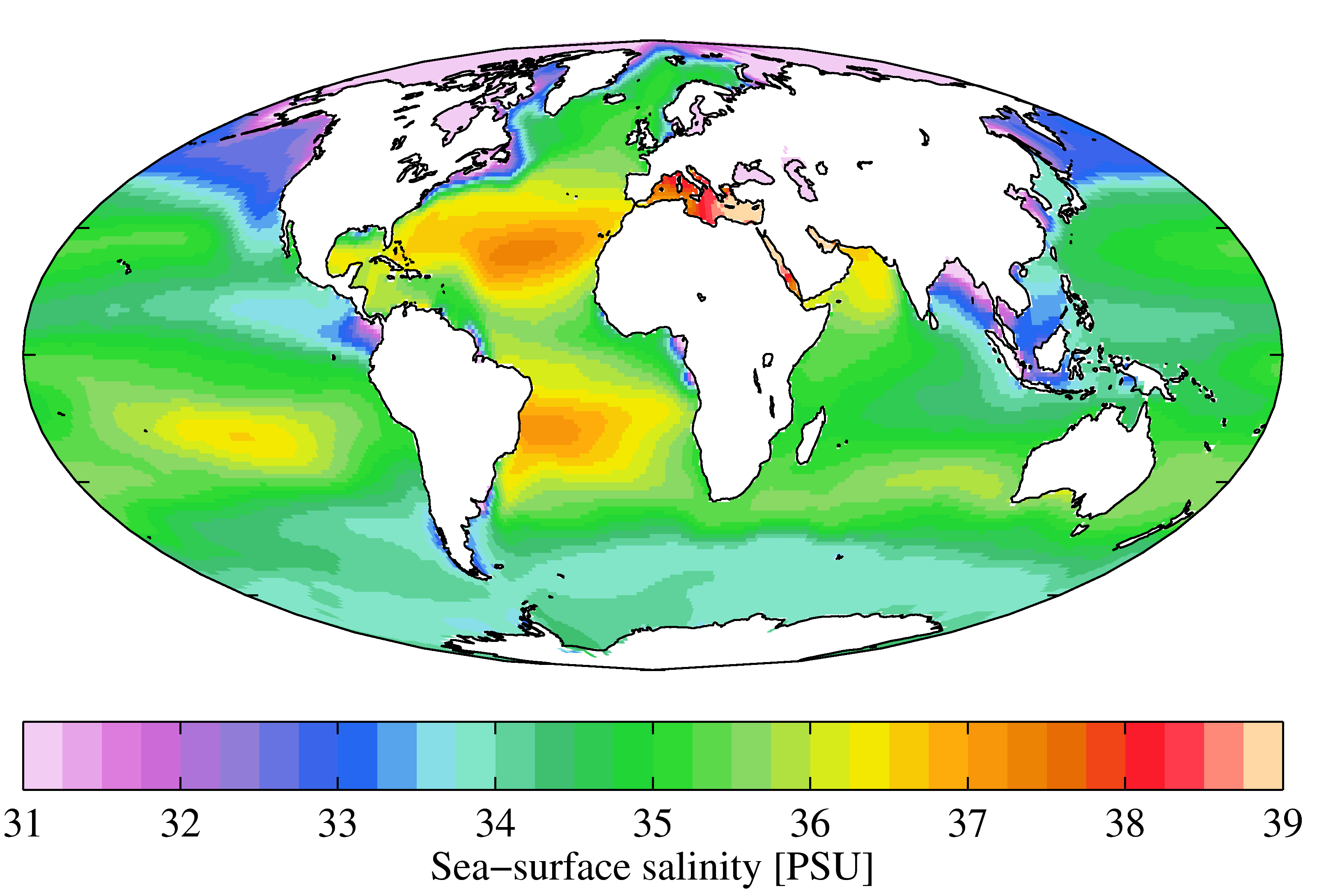
Oligohaline
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Foam
Foams are materials formed by trapping pockets of gas in a liquid or solid. A bath sponge and the head on a glass of beer are examples of foams. In most foams, the volume of gas is large, with thin films of liquid or solid separating the regions of gas. Soap foams are also known as suds. Solid foams can be closed-cell or open-cell. In closed-cell foam, the gas forms discrete pockets, each completely surrounded by the solid material. In open-cell foam, gas pockets connect to each other. A bath sponge is an example of an open-cell foam: water easily flows through the entire structure, displacing the air. A sleeping mat is an example of a closed-cell foam: gas pockets are sealed from each other so the mat cannot soak up water. Foams are examples of dispersed media. In general, gas is present, so it divides into gas bubbles of different sizes (i.e., the material is polydisperse)—separated by liquid regions that may form films, thinner and thinner when the liquid phase drain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymictic Lake
Polymictic lakes are holomictic lakes that are too shallow to develop thermal stratification; thus, their waters can mix from top to bottom throughout the ice-free period. Polymictic lakes can be divided into cold polymictic lakes (i.e., those that are ice-covered in winter), and warm polymictic lakes (i.e., polymictic lakes in regions where ice-cover does not develop in winter). While such lakes are well-mixed on average, during low-wind periods, weak and ephemeral stratification can often develop. See also * Amictic lake * Holomictic lake * Meromictic lake * Monomictic lake * Dimictic lake * Thermocline A thermocline (also known as the thermal layer or the metalimnion in lakes) is a thin but distinct layer in a large body of fluid (e.g. water, as in an ocean or lake; or air, e.g. an atmosphere) in which temperature changes more drastically with ... * References External links "Circulation: annual patterns of dimictic lakes" at Encyclopædia Britannica Online Lakes by t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |