|
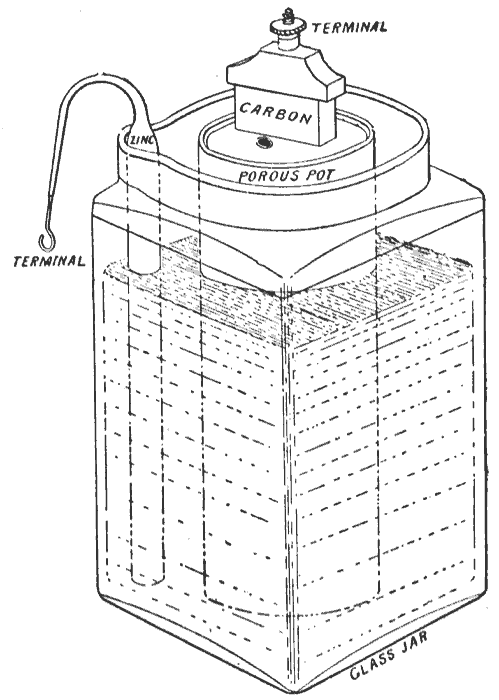
Leclanché Cell
The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode (positive terminal) of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode (negative terminal) of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell. History In 1866, Georges Leclanché invented a battery that consisted of a zinc anode and a manganese dioxide cathode wrapped in a porous material, dipped in a jar of ammonium chloride solution. The manganese dioxide cathode had a little carbon mixed into it as well, which improved conductivity and absorption. It provided a voltage of 1.4 volts.Simms, J.W. (1965) ''The Boy Electrician'' M.I.E.E. p. 61 This cell achieved very quick success in telegraphy, signalling and electric bell work. The dry cell form was used to power early telephones—usually from an adjacent woo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophile
A hydrophile is a molecule or other molecular entity that is attracted to water molecules and tends to be dissolved by water.Liddell, H.G. & Scott, R. (1940). ''A Greek-English Lexicon'' Oxford: Clarendon Press. In contrast, hydrophobes are not attracted to water and may seem to be repelled by it. Hygroscopics ''are'' attracted to water, but are not dissolved by water. Molecules A hydrophilic molecule or portion of a molecule is one whose interactions with water and other polar substances are more thermodynamically favorable than their interactions with oil or other hydrophobic solvents. They are typically charge-polarized and capable of hydrogen bonding. This makes these molecules soluble not only in water but also in other polar solvents. Hydrophilic molecules (and portions of molecules) can be contrasted with hydrophobic molecules (and portions of molecules). In some cases, both hydrophilic and hydrophobic properties occur in a single molecule. An example of these amphiph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromotive Force
In electromagnetism and electronics, electromotive force (also electromotance, abbreviated emf, denoted \mathcal or ) is an energy transfer to an electric circuit per unit of electric charge, measured in volts. Devices called electrical ''transducers'' provide an emf by converting other forms of energy into electrical energy. Other electrical equipment also produce an emf, such as batteries, which convert chemical energy, and generators, which convert mechanical energy. This energy conversion is achieved by physical forces applying physical work on electric charges. However, electromotive force itself is not a physical force, and for the current ISO/IEC standards consider the term deprecated, favoring the names source voltage or source tension instead (denoted U_s). An electronic–hydraulic analogy may view emf as the mechanical work done to water by a pump, which results in a pressure difference (analogous to voltage). In electromagnetic induction, emf can be defined ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquioxide
A sesquioxide is an oxide of an element (or radical), where the ratio between the number of atoms of that element and the number of atoms of oxygen is 2:3. For example, aluminium oxide and phosphorus(III) oxide are sesquioxides. Many sesquioxides contain a metal in the +3 oxidation state and the oxide ion , e.g., aluminium oxide , lanthanum(III) oxide and iron(III) oxide . Sesquioxides of iron and aluminium are found in soil. The alkali metal sesquioxides are exceptions because they contain both peroxide and superoxide ions, e.g., rubidium sesquioxide is formulated . Sesquioxides of metalloids and nonmetals are exceptions too, e.g. boron trioxide , dinitrogen trioxide and phosphorus(III) oxide . Many transition metal oxides crystallize in the corundum structure type, with space group Rc. Sesquioxides of rare earth elements crystalize into one or more of three crystal structures: hexagonal (type A, space group Pm1), monoclinic (type B, space group C2/m), or body-centered cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxide Ion
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently bound group –OH of atoms is the hydroxy group. Both the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. Many inorganic substances which bear the word ''hydroxide'' in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. Hydroxide ion The hydroxide ion is a natural part of wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Oxide
Manganese oxide is any of a variety of manganese oxides and hydroxides.Wells A.F. (1984) ''Structural inorganic chemistry'' 5th edition Oxford Science Publications, . These include * Manganese(II) oxide, MnO * Manganese(II,III) oxide, Mn3O4 * Manganese(III) oxide, Mn2O3 * Manganese dioxide, MnO2 * Manganese(VI) oxide, MnO3 * Manganese(VII) oxide, Mn2O7 Other manganese oxides include Mn5O8, Mn7O12 and Mn7O13. Minerals It may refer more specifically to the following manganese minerals: * Birnessite * Buserite * Hausmannite * Manganite * Manganosite * Psilomelane * Pyrolusite Manganese may also form mixed oxides with other metals : * Bixbyite, a manganese iron oxide mineral * Jacobsite, a manganese iron oxide mineral * Columbite * Tantalite, a mineral group close to columbite * Coltan, a mixture of columbite and tantalite * Galaxite, a spinel mineral * Todorokite Todorokite is a rare complex hydrous manganese oxide mineral with the chemical formula . It was named in 1934 f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a test charge between the two points. In the International System of Units, the derived unit for voltage is named ''volt''. The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in generator, inductors, and transformers). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. A voltmeter can be used to measure the voltage between two points in a system. Often a common reference potential such as the ground of the system is used as one of the points. A voltage can represent either a source ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a test charge between the two points. In the International System of Units, the derived unit for voltage is named ''volt''. The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in generator, inductors, and transformers). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. A voltmeter can be used to measure the voltage between two points in a system. Often a common reference potential such as the ground of the system is used as one of the points. A voltage can represent either a source ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Current
An electric current is a stream of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is measured as the net rate of flow of electric charge through a surface or into a control volume. The moving particles are called charge carriers, which may be one of several types of particles, depending on the conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or holes. In an electrolyte the charge carriers are ions, while in plasma, an ionized gas, they are ions and electrons. The SI unit of electric current is the ampere, or ''amp'', which is the flow of electric charge across a surface at the rate of one coulomb per second. The ampere (symbol: A) is an SI base unit. Electric current is measured using a device called an ammeter. Electric currents create magnetic fields, which are used in motors, generators, inductors, and transformers. In ordinary con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Circuit
An electrical network is an interconnection of electrical components (e.g., batteries, resistors, inductors, capacitors, switches, transistors) or a model of such an interconnection, consisting of electrical elements (e.g., voltage sources, current sources, resistances, inductances, capacitances). An electrical circuit is a network consisting of a closed loop, giving a return path for the current. Linear electrical networks, a special type consisting only of sources (voltage or current), linear lumped elements (resistors, capacitors, inductors), and linear distributed elements (transmission lines), have the property that signals are linearly superimposable. They are thus more easily analyzed, using powerful frequency domain methods such as Laplace transforms, to determine DC response, AC response, and transient response. A resistive circuit is a circuit containing only resistors and ideal current and voltage sources. Analysis of resistive circuits is less complicated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3D-balls.png)



