|
Late-stage Functionalization
Late-stage functionalization (LSF) is a desired, chemical or biochemical, Chemoselectivity, chemoselective Chemical reaction, transformation on a complex molecule to provide at least one analog in sufficient quantity and purity for a given purpose without needing the addition of a functional group that exclusively serves to enable said transformation. Molecular complexity is an intrinsic property of each molecule and frequently determines the synthetic effort to make it. LSF can significantly diminish this synthetic effort, and thus enables access to molecules, which would otherwise not be available or too difficult to access. The requirements for LSF can be met by both C-H functionalization, C–H functionalization reactions and functional group manipulations. LSF reactions are particularly relevant and often used in the fields of drug discovery and Materials Chemistry, materials chemistry, although no LSF has been implemented in a commercial process. Chemoselectivity All LSF ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemoselectivity
Chemoselectivity is the preferential outcome of a chemical reaction over a set of possible alternative reactions. In another definition, chemoselectivity refers to the selective reactivity of one functional group in the presence of others; often this process in convoluted and protecting groups are on the molecular connectivity alone. Such predictions based on connectivity are generally considered plausible, but the physical outcome of the actual reaction is ultimately dependent on a number of factors that are practically impossible to predict to any useful accuracy (solvent, atomic orbitals, etc.). Chemoselectivity can be difficult to predict, but observing selective outcomes in cases where many reactions are plausible, is common. Examples include the selective organic Redox, reduction of the greater relative chemoselectivity of sodium borohydride Redox, reduction versus lithium aluminium hydride Redox, reduction. In another example, the compound 4-methoxyacetophenone is oxidized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Synthesis
As a topic of chemistry, chemical synthesis (or combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses, the process is reproducible and reliable. A chemical synthesis involves one or more compounds (known as '' reagents'' or ''reactants'') that will experience a transformation when subjected to certain conditions. Various reaction types can be applied to formulate a desired product. This requires mixing the compounds in a reaction vessel, such as a chemical reactor or a simple round-bottom flask. Many reactions require some form of processing (" work-up") or purification procedure to isolate the final product. The amount produced by chemical synthesis is known as the ''reaction yield''. Typically, yields are expressed as a mass in grams (in a laboratory setting) or as a percentage of the total theoretical quantity that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Constitutional Isomer
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a chemical compound, compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct chemical bond, bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regioselectivity
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where on a substituted benzene ring a further substituent will be added. A specific example is a halohydrin formation reaction with 2-propenylbenzene: : Because of the preference for the formation of one product over another, the reaction is selective. This reaction is regioselective because it selectively generates one constitutional isomer rather than the other. Various examples of regioselectivity have been formulated as rules for certain classes of compounds under certain conditions, many of which are named. Among the first introduced to chemistry students are Markovnikov's rule for the addition of protic acids to alkenes, and the Fürst-Plattner rule for the addition of nucleophiles to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Example For A Site-unselective LSF
Example may refer to: * '' exempli gratia'' (e.g.), usually read out in English as "for example" * .example, reserved as a domain name that may not be installed as a top-level domain of the Internet ** example.com, example.net, example.org, example.edu, second-level domain names reserved for use in documentation as examples * HMS ''Example'' (P165), an Archer-class patrol and training vessel of the Royal Navy Arts * '' The Example'', a 1634 play by James Shirley * ''The Example'' (comics), a 2009 graphic novel by Tom Taylor and Colin Wilson * Example (musician) Elliot John Gleave (born 20 June 1982), better known by his stage name Example, is an English musician, singer, songwriter, rapper and record producer. His name arose due to his initials being E.G., which is an abbreviation of the Latin phrase ..., the British dance musician Elliot John Gleave (born 1982) * ''Example'' (album), a 1995 album by American rock band For Squirrels See also * * Exemplar (disamb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
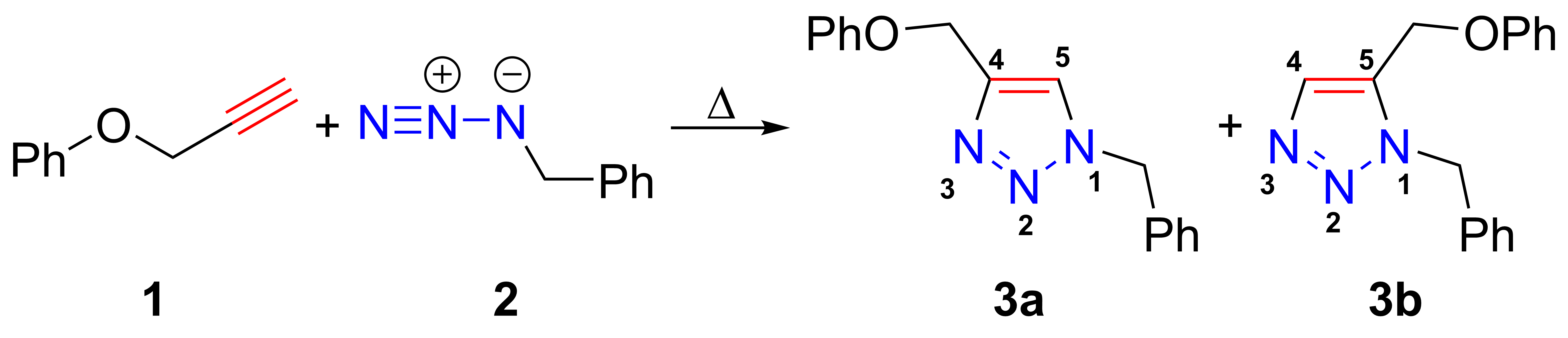
Huisgen Cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
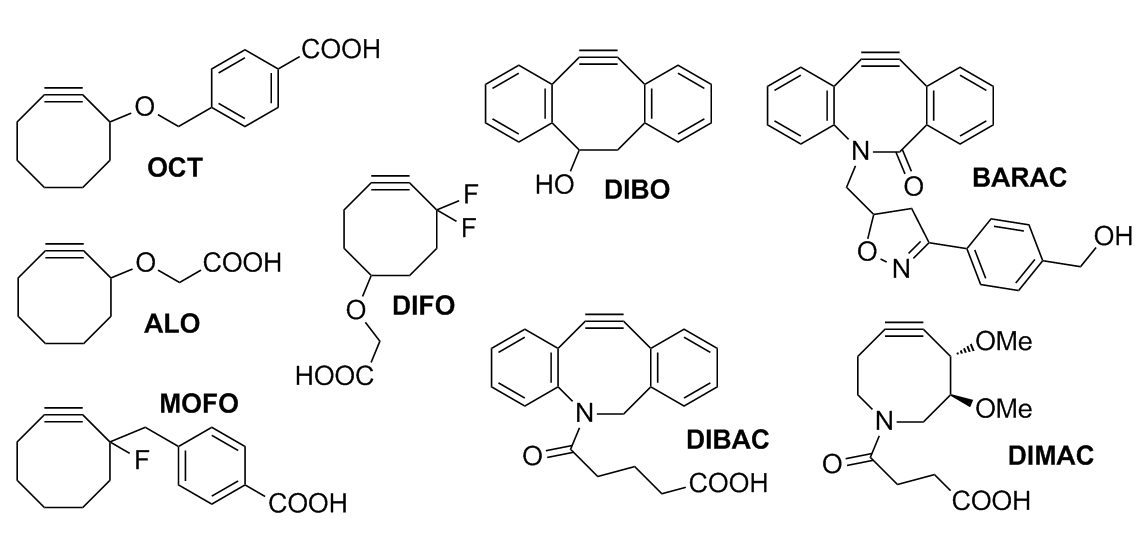
Copper-free Click Chemistry
Copper-free click chemistry is a bio-orthogonal reaction as a variant of an azide-alkyne Huisgen cycloaddition. By eliminating cytotoxic copper catalysts, the reaction proceeds without live-cell toxicity. It was developed as a faster alternative to the Staudinger ligation with the first generation of Cu-free click chemistry, producing rate constants over 63 times faster. Although the reaction produces a regioisomeric mixture of triazoles, the lack of regioselectivity in the reaction is not a major concern for its applications in bioorthogonal chemistry. More regiospecific and less bio-orthogonal requirements are best served by the traditional Huisgen cycloaddition, especially given the low yield and synthetic difficulty of synthesizing a strained cyclooctyne (compared to the addition of a terminal alkyne). The bio-orthogonality of the reaction has allowed the Cu-free click reaction to be applied within cultured cells, live zebrafish, and mice. The absence of exogenous metal cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioorthogonal Chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation. The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |