|
Lyc Photon
Lyman continuum photons (abbrev. LyC), shortened to Ly continuum photons or Lyc photons, are the photons emitted from stars or active galactic nuclei at photon energies above the Lyman limit. Hydrogen is ionized by absorbing LyC. Working from Victor Schumann's discovery of ultraviolet light, from 1906 to 1914, Theodore Lyman observed that at wavelengths above the limit atomic hydrogen absorbs light only at specific frequencies (or wavelengths) and the Lyman series is thus named after him. All the wavelengths in the Lyman series are in the ultraviolet band. This quantized absorption behavior occurs only up to an energy limit, known as the ionization energy. In the case of neutral atomic hydrogen, the minimum ionization energy is equal to the Lyman limit, where the photon has enough energy to completely ionize the atom, resulting in a free proton and a free electron. Above this energy (below this wavelength), ''all'' wavelengths of light may be absorbed. This forms a continuum in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can move no faster than the speed of light measured in vacuum. The photon belongs to the class of boson particles. As with other elementary particles, photons are best explained by quantum mechanics and exhibit wave–particle duality, their behavior featuring properties of both waves and particles. The modern photon concept originated during the first two decades of the 20th century with the work of Albert Einstein, who built upon the research of Max Planck. While Planck was trying to explain how matter and electromagnetic radiation could be in thermal equilibrium with one another, he proposed that the energy stored within a material object should be regarded as composed of an integer number of discrete, equal-sized parts. To explain the pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up quark, up and down quark, down quarks. Electrons are extremely lightweight particles that orbit the positively charged atomic nucleus, nucleus of atoms. Their negative charge is balanced by the positive charge of protons in the nucleus, giving atoms their overall electric charge#Charge neutrality, neutral charge. Ordinary matter is composed of atoms, each consisting of a positively charged nucleus surrounded by a number of orbiting electrons equal to the number of protons. The configuration and energy levels of these orbiting electrons determine the chemical properties of an atom. Electrons are bound to the nucleus to different degrees. The outermost or valence electron, valence electrons are the least tightly bound and are responsible for th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Balmer Series
The Balmer series, or Balmer lines in atomic physics, is one of a set of hydrogen spectral series, six named series describing the spectral line emissions of the hydrogen atom. The Balmer series is calculated using the Balmer formula, an empirical equation discovered by Johann Balmer in 1885. The visible spectrum of light from hydrogen displays four wavelengths, 410 metre#SI prefixed forms of metre, nm, 434 nm, 486 nm, and 656 nm, that correspond to emissions of photons by electrons in excited states transitioning to the quantum level described by the principal quantum number ''n'' equals 2. There are several prominent ultraviolet Balmer lines with wavelengths shorter than 400 nm. The series continues with an infinite number of lines whose wavelengths asymptotically approach the limit of 364.5 nm in the ultraviolet. After Balmer's discovery, five other hydrogen spectral series were discovered, corresponding to electrons transitioning to values of ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoionization
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule. Cross section Not every interaction between a photon and an atom, or molecule, will result in photoionization. The probability of photoionization is related to the photoionization cross section of the species – the probability of an ionization event conceptualized as a hypothetical cross-sectional area. This cross section depends on the energy of the photon (proportional to its wavenumber) and the species being considered i.e. it depends on the structure of the molecular species. In the case of molecules, the photoionization cross-section can be estimated by examination of Franck-Condon factors between a ground-state molecule and the target ion. This can be initialized by computing the vibrations of a molecule and associated cation (post ionization) using quantum chemical software e.g. QChem. For photon energies below the ionization threshold, the photoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinetic Energy
In physics, the kinetic energy of an object is the form of energy that it possesses due to its motion. In classical mechanics, the kinetic energy of a non-rotating object of mass ''m'' traveling at a speed ''v'' is \fracmv^2.Resnick, Robert and Halliday, David (1960) ''Physics'', Section 7-5, Wiley International Edition The kinetic energy of an object is equal to the work, or force ( F) in the direction of motion times its displacement ( s), needed to accelerate the object from rest to its given speed. The same amount of work is done by the object when decelerating from its current speed to a state of rest. The SI unit of energy is the joule, while the English unit of energy is the foot-pound. In relativistic mechanics, \fracmv^2 is a good approximation of kinetic energy only when ''v'' is much less than the speed of light. History and etymology The adjective ''kinetic'' has its roots in the Greek word κίνησις ''kinesis'', meaning "motion". The dichoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosphere
The photosphere is a star's outer shell from which light is radiated. It extends into a star's surface until the plasma becomes opaque, equivalent to an optical depth of approximately , or equivalently, a depth from which 50% of light will escape without being scattered. A photosphere is the region of a luminous object, usually a star, that is transparent to photons of certain wavelengths. Stars, except neutron stars, have no solid or liquid surface. Therefore, the photosphere is typically used to describe the Sun's or another star's visual surface. Etymology The term ''photosphere'' is derived from Ancient Greek roots, φῶς, φωτός/''phos'', ''photos'' meaning "light" and σφαῖρα/''sphaira'' meaning "sphere", in reference to it being a spherical surface that is perceived to emit light. Temperature The surface of a star is defined to have a temperature given by the effective temperature in the Stefan–Boltzmann law. Various stars have photospheres of vari ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gamma-ray Astronomy
Gamma-ray astronomy is a subfield of astronomy where scientists observe and study celestial objects and phenomena in outer space which emit cosmic electromagnetic radiation in the form of gamma rays,Astronomical literature generally hyphenates "gamma-ray" when used as an adjective, but uses "gamma ray" without a hyphen for the noun. i.e. photons with the highest energies (above 100 keV) at the very shortest wavelengths. Radiation below 100 keV is classified as X-rays and is the subject of X-ray astronomy. In most cases, gamma rays from solar flares and Earth's atmosphere fall in the MeV range, but it's now known that solar flares can also produce gamma rays in the GeV range, contrary to previous beliefs. Much of the detected gamma radiation stems from collisions between hydrogen gas and cosmic rays within our galaxy. These gamma rays, originating from diverse mechanisms such as electron-positron annihilation, the inverse Compton effect and in some cases gamma decay ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Astronomy
X-ray astronomy is an observational branch of astronomy which deals with the study of X-ray observation and detection from astronomical objects. X-radiation is absorbed by the Earth's atmosphere, so instruments to detect X-rays must be taken to high altitude by Balloon-borne telescope, balloons, sounding rockets, and X-ray astronomy satellite, satellites. X-ray astronomy uses a type of space telescope that can see x-ray radiation which standard optical telescopes, such as the Mauna Kea Observatories, cannot. X-ray generation, X-ray emission is expected from astronomical objects that contain extremely hot gases at temperatures from about a million kelvin (K) to hundreds of millions of kelvin (MK). Moreover, the maintenance of the E-layer of ionized gas high in the Earth's thermosphere also suggested a strong extraterrestrial source of X-rays. Although theory predicted that the Sun and the stars would be prominent X-ray sources, there was no way to verify this because Earth's atmo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
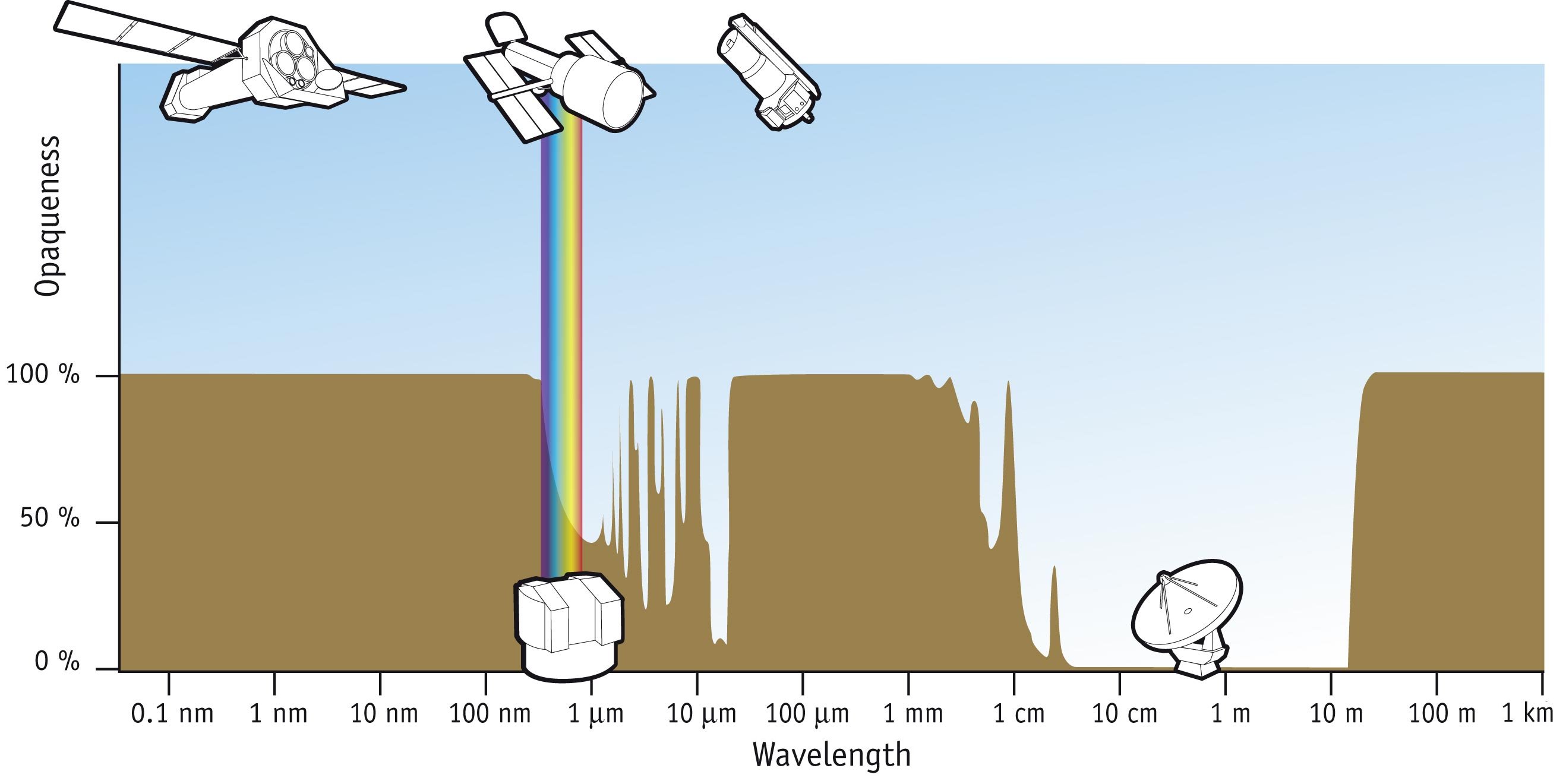
Electromagnetic Spectrum
The electromagnetic spectrum is the full range of electromagnetic radiation, organized by frequency or wavelength. The spectrum is divided into separate bands, with different names for the electromagnetic waves within each band. From low to high frequency these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. Radio waves, at the low-frequency end of the spectrum, have the lowest photon energy and the longest wavelengths—thousands of kilometers, or more. They can be emitted and received by antenna (radio), antennas, and pass through the atmosphere, foliage, and most building materials. Gamma rays, at the high-frequency end of the spectrum, have the highest photon energies and the shortest wavelengths—much smaller than an atomic nucleus. Gamma rays, X-rays, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs, Cherenkov radiation, and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. The photons of ultraviolet have greater energy than those of visible light, from about 3.1 to 12 electron volts, around the minimum energy required to ionize atoms. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack sufficient energy, it can induce chemical reactions and cause many substances to glow or fluoresce. Many practical applications, including chemical and biological effects, are derived from the way that UV radiation can interact with organic molecules. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum. When used as a Units of energy, unit of energy, the numerical value of 1 eV in joules (symbol J) is equal to the numerical value of the Electric charge, charge of an electron in coulombs (symbol C). Under the 2019 revision of the SI, this sets 1 eV equal to the exact value Historically, the electronvolt was devised as a standard unit of measure through its usefulness in Particle accelerator#Electrostatic particle accelerators, electrostatic particle accelerator sciences, because a particle with electric charge ''q'' gains an energy after passing through a voltage of ''V''. Definition and use An electronvolt is the amount of energy gained or lost by a single electron when it moves through an Voltage, electric potential differenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gigahertz
The hertz (symbol: Hz) is the unit of frequency in the International System of Units (SI), often described as being equivalent to one event (or cycle) per second. The hertz is an SI derived unit whose formal expression in terms of SI base units is 1/s or s−1, meaning that one hertz is one per second or the reciprocal of one second. It is used only in the case of periodic events. It is named after Heinrich Rudolf Hertz (1857–1894), the first person to provide conclusive proof of the existence of electromagnetic waves. For high frequencies, the unit is commonly expressed in multiples: kilohertz (kHz), megahertz (MHz), gigahertz (GHz), terahertz (THz). Some of the unit's most common uses are in the description of periodic waveforms and musical tones, particularly those used in radio- and audio-related applications. It is also used to describe the clock speeds at which computers and other electronics are driven. The units are sometimes also used as a representation of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |