|
Lithium Succinate
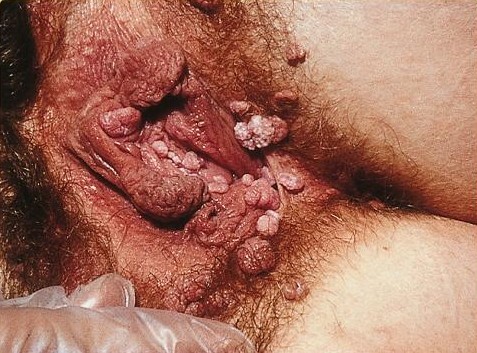
Lithium succinate (C4H4Li2O4), the dilithium salt of succinic acid, is a drug used in the treatment of seborrhoeic dermatitis and proposed for the treatment of anogenital warts Genital warts are a sexually transmitted infection caused by certain types of human papillomavirus (HPV). They are generally pink in color and project out from the surface of the skin. Usually they cause few symptoms, but can occasionally be pa .... References External links * Lithium salts Organolithium compounds Succinates {{dermatologic-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptides, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Succinic Acid
Succinic acid () is a dicarboxylic acid with the chemical formula (CH2)2(CO2H)2. The name derives from Latin ''succinum'', meaning amber. In living organisms, succinic acid takes the form of an anion, succinate, which has multiple biological roles as a metabolic intermediate being converted into fumarate by the enzyme succinate dehydrogenase in complex 2 of the electron transport chain which is involved in making ATP, and as a signaling molecule reflecting the cellular metabolic state. It is marketed as food additive E363. Succinate is generated in mitochondria via the tricarboxylic acid cycle (TCA). Succinate can exit the mitochondrial matrix and function in the cytoplasm as well as the extracellular space, changing gene expression patterns, modulating epigenetic landscape or demonstrating hormone-like signaling. As such, succinate links cellular metabolism, especially ATP formation, to the regulation of cellular function. Dysregulation of succinate synthesis, and therefore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seborrhoeic Dermatitis
Seborrhoeic dermatitis, sometimes inaccurately referred to as seborrhoea, is a long-term skin disorder. Symptoms include red, scaly, greasy, itchy, and inflamed skin. Areas of the skin rich in oil-producing glands are often affected including the scalp, face, and chest. It can result in social or self-esteem problems. In babies, when the scalp is primarily involved, it is called cradle cap. Dandruff is a milder form of the condition without inflammation. The cause is unclear but believed to involve a number of genetic and environmental factors. Risk factors include poor immune function, Parkinson's disease, and alcoholic pancreatitis. The condition may worsen with stress or during the winter. The '' Malassezia'' yeast is believed to play a role. It is not a result of poor hygiene. Diagnosis is typically based on the symptoms. The condition is not contagious. The typical treatment is antifungal cream and anti-inflammatory agents. Specifically, ketoconazole or ciclopirox are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anogenital Warts
Genital warts are a sexually transmitted infection caused by certain types of human papillomavirus (HPV). They are generally pink in color and project out from the surface of the skin. Usually they cause few symptoms, but can occasionally be painful. Typically they appear one to eight months following exposure. Warts are the most easily recognized symptom of genital HPV infection. HPV types 6 and 11 are responsible for causing majority of genital warts whereas HPV types 16, 18, 31, 33, and 35 are also occasionally found. It is spread through direct skin-to-skin contact, usually during oral, genital, or anal sex with an infected partner. Diagnosis is generally based on symptoms and can be confirmed by biopsy. The types of HPV that cause cancer are not the same as those that cause warts. Some HPV vaccines can prevent genital warts as may condoms. Treatment options include creams such as podophyllin, imiquimod, and trichloroacetic acid. Cryotherapy or surgery may also b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Salts
Lithium (from el, λίθος, lithos, lit=stone) is a chemical element with the symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard conditions, it is the least dense metal and the least dense solid element. Like all alkali metals, lithium is highly reactive and flammable, and must be stored in vacuum, inert atmosphere, or inert liquid such as purified kerosene or mineral oil. When cut, it exhibits a metallic luster, but moist air corrodes it quickly to a dull silvery gray, then black tarnish. It never occurs freely in nature, but only in (usually ionic) compounds, such as pegmatitic minerals, which were once the main source of lithium. Due to its solubility as an ion, it is present in ocean water and is commonly obtained from brines. Lithium metal is isolated electrolytically from a mixture of lithium chloride and potassium chloride. The nucleus of the lithium atom verges on instability, since the two stable lithium isotopes fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organolithium Compounds
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |