|
List Of Esters
In chemistry, an ester is a chemical compound, compound derived from an acid (organic or inorganic) in which the hydrogen atom (H) of at least one Acid, acidic hydroxyl group () of that acid is replaced by an organyl group (). Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well (i.e. esters of acidic , , , and groups). According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. An example of an ester formation is the substitution reaction between a carboxylic acid () and an Alcohol (chemistry), alcohol (R'OH), forming an ester (), where R and R′ are organyl groups, or hydrogen, H in the case of esters of formic acid. Glycerides, which are fatty acid esters of glycerol, are important esters in biology, being one of the main classes of lipids, and making up the bulk of animal fats and vegetable oils. Esters of carboxylic acids with low molecular we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substitution Reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent. A good example of a substitution reaction is halogenation. When chlorine gas (Cl2) is irradiated, some of the molecules are split into two chlorine radicals (Cl•), whose free electrons are strongly n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing. Polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties. Cotton-polyester blends can be strong, wrinkle- and tear-resistant, and reduce shrinking. Synthetic fibers using polyester have high water, wind and environmental resistance compared to plant-derived fibers. They are less Fireproofing, fire-resistant and can melt when ignited. Liquid crystalline polyesters are among the first industrially used liquid crystal polymers. They are use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroglycerin
Nitroglycerin (NG), (alternative spelling of nitroglycerine) also known as trinitroglycerin (TNG), nitro, glyceryl trinitrate (GTN), or 1,2,3-trinitroxypropane, is a dense, colorless, oily, explosive liquid most commonly produced by nitrating glycerol with white fuming nitric acid under conditions appropriate to the formation of the nitric acid ester. Chemically, the substance is an organic nitrate compound rather than a nitro compound, but the traditional name is retained. Invented in 1847 by Ascanio Sobrero, nitroglycerin has been used ever since as an active ingredient in the manufacture of explosives, namely dynamite, and as such it is employed in the construction, demolition, and mining industries. Since the 1880s, it has been used by militaries as an active ingredient and gelatinizer for nitrocellulose in some solid propellants such as cordite and ballistite. It is a major component in double-based smokeless propellants used by reloaders. Combined with nitrocellulose, hund ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate Ester
In organic chemistry, a nitrate ester is an organic functional group with the formula , where R stands for any organic residue. They are the esters of nitric acid and alcohols. A well-known example is nitroglycerin, which is not a ''nitro'' compound, despite its name. : Synthesis and reactions Nitrate esters are typically prepared by condensation of nitric acid and the alcohol: For example, the simplest nitrate ester, methyl nitrate, is formed by reaction of methanol and nitric acid in the presence of sulfuric acid: :CH3OH + HNO3 -> CH3ONO2 + H2O This condensation is sometimes called "nitroxylation". Explosive properties The thermal decomposition of nitrate esters mainly yields the gases molecular nitrogen (N2) and carbon dioxide. The considerable chemical energy of the detonation is due to the high strength of the bond in molecular nitrogen. This stoichiometry is illustrated by the equation for the detonation of nitroglycerin. : Illustrative of the highly sensitive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoester
In chemistry, a phosphodiester bond occurs when exactly two of the hydroxyl groups () in phosphoric acid react with hydroxyl groups on other molecules to form two ester bonds. The "bond" involves this linkage . Discussion of phosphodiesters is dominated by their prevalence in DNA and RNA, but phosphodiesters occur in other biomolecules, e.g. acyl carrier proteins. Phosphodiester bonds make up the backbones of DNA and RNA. The phosphate is attached to the 5' carbon. The 3' carbon of one sugar is bonded to the 5' phosphate of the adjacent sugar. Specifically, the phosphodiester bond links the 3' carbon atom of one sugar molecule and the 5' carbon atom of another (hence the name, 3', 5' phosphodiester linkage). These saccharide groups are derived from deoxyribose in DNA and ribose in RNA. Phosphodiesters are negatively charged at pH 7. Repulsion between these negative charges influences the conformation of the polynucleic acids. The negative charge attracts histones, metal c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pheromone
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavior of the receiving individuals. There are ''alarm signal, alarm pheromones'', ''food trail pheromones'', ''sex pheromones'', and many others that affect behavior or physiology. Pheromones are used by many organisms, from basic unicellular prokaryotes to complex multicellular eukaryotes. Their use among insects has been particularly well documented. In addition, some vertebrates, plants and ciliates communicate by using pheromones. The ecological functions and evolution of pheromones are a major topic of research in the field of chemical ecology. Background The portmanteau word "pheromone" was coined by Peter Karlson and Martin Lüscher in 1959, based on the Greek φερω ''pheroo'' ('I carry') and ὁρμων ''hormon'' ('stimulating'). P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragrance—the characteristic fragrance of the plant from which it is derived. The term "essential" used here does ''not'' mean indispensable or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism. Essential oils are generally extracted by distillation, often by using steam. Other processes include expression, solvent extraction, '' sfumatura'', absolute oil extraction, resin tapping, wax embedding, and cold pressing. They are used in perfumes, cosmetics, soaps, air ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipids
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
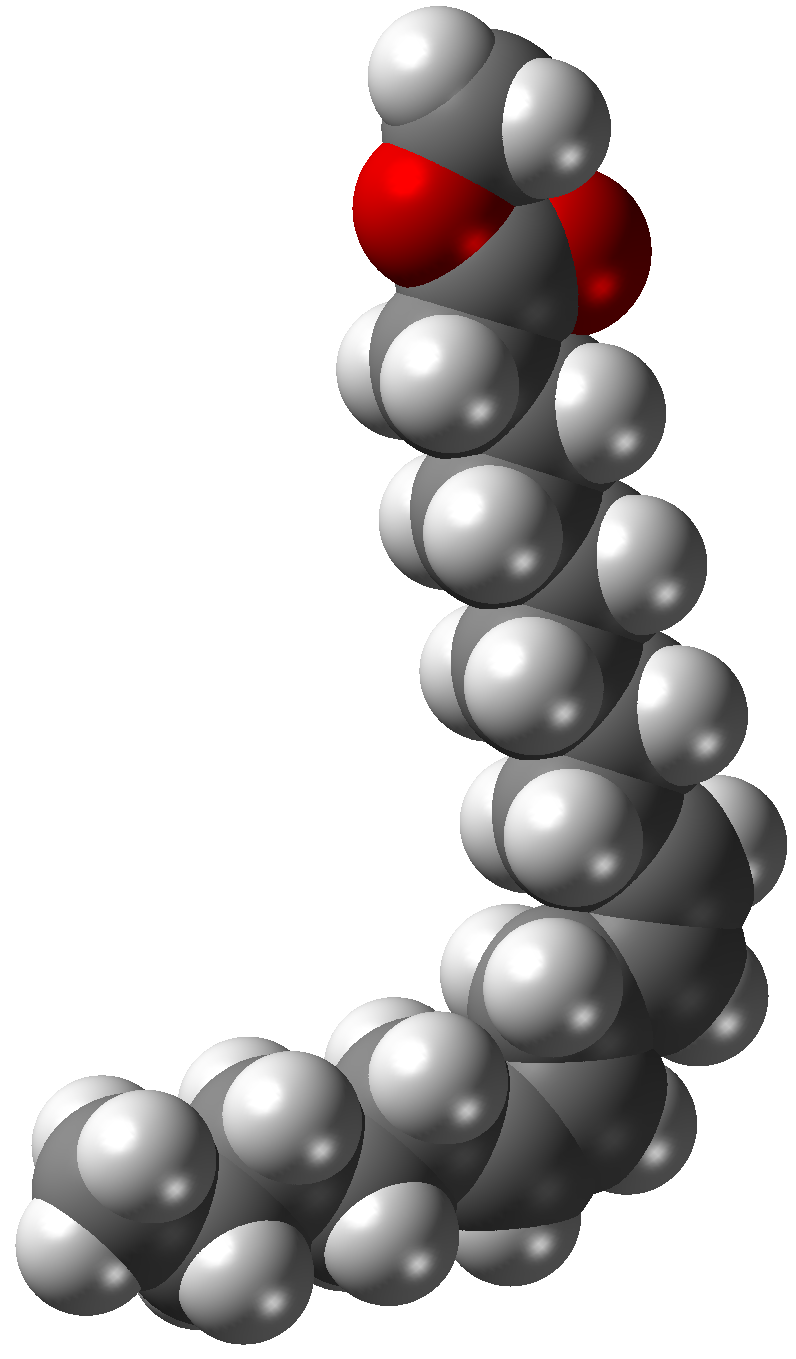
Fatty Acid Ester
Fatty acid esters (FAEs) are a type of ester that result from the combination of a fatty acid with an alcohol. When the alcohol component is glycerol, the fatty acid esters produced can be monoglycerides, diglycerides, or triglycerides. Dietary fats are chemically triglycerides. Esters of fatty acids are colorless, although degraded samples are sometime appear to be yellow or even brown. The triglycerides are powders, flakes, coarse powders, or granular or waxy lumps, oils or liquids. They are almost odorless. Biodiesels are typically fatty acid esters made by the transesterification of vegetable fats and oils. In this process the glycerol component is replaced with a different alcohol. The most commonly used alcohol is methanol, producing fatty acid methyl esters (FAME). When ethanol is used fatty acid ethyl esters (FAEE) are created. Other alcohols used for the production of biodiesel include butanol and isopropanol. Fatty acid ethyl esters are biomarkers for the consumption o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyceride
Glycerides, more correctly known as acylglycerols, are esters formed from glycerol and fatty acids, and are generally very hydrophobic. Glycerol has three hydroxyl functional groups, which can be esterified with one, two, or three fatty acids to form mono-, di-, and triglycerides. These structures vary in their fatty acid alkyl groups as they can contain different carbon numbers, different degrees of unsaturation, and different configurations and positions of olefins. Vegetable oils and animal fats contain mostly triglycerides, but are broken down by natural enzymes (lipases) into mono and diglycerides and free fatty acids and glycerol. Soaps are formed from the reaction of glycerides with sodium hydroxide. The product of the reaction is glycerol and salts of fatty acids. Fatty acids in the soap emulsify the oils in dirt, enabling the removal of oily dirt with water. Partial glycerides are esters of glycerol with fatty acids, where not all the hydroxyl groups are esterified ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |