|
Lifileucel
Lifileucel, sold under the brand name Amtagvi, is an Adoptive T-cell therapy, adoptive T cell therapy used for the treatment of melanoma. Specifically, lifileucel is a tumor-derived T cell immunotherapy composed of a recipient's own T cells. A portion of the recipient's tumor tissue is removed during a surgical procedure prior to treatment. The recipient's T cells (the tumor-infiltrating lymphocytes) are separated from the tumor tissue, multiplied and then infused into the patient in a single dose. T cells are a type of cell that helps the immune system fight cancer and infections. Lifileucel is the first tumor-derived T cell immunotherapy approved by the US Food and Drug Administration (FDA). It was approved for medical use in the United States in February 2024. Medical uses Lifileucel is indicated for the treatment of adults with unresectable (unable to be removed with surgery) or metastatic (spread to other parts of the body) melanoma previously treated with other the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
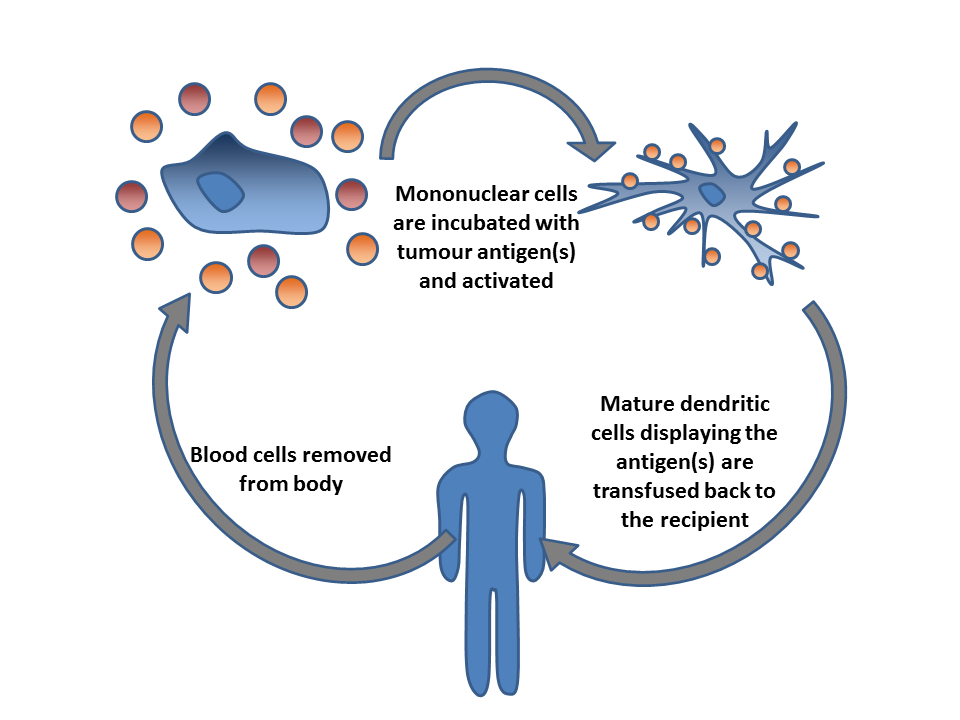
Adoptive T-cell Therapy
Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology. Cancer immunotherapy exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can bind to antibody proteins or T-cell receptors, triggering an immune system response. The tumor antigens are often proteins or other macromolecules (e.g., carbohydrates). Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill. The clinical success of cancer immunotherapy is highly variable between different forms of cancer; for instance, certain subtypes of gastric cancer react well to the approach whereas immunotherapy is not effec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanoma
Melanoma is the most dangerous type of skin cancer; it develops from the melanin-producing cells known as melanocytes. It typically occurs in the skin, but may rarely occur in the mouth, intestines, or eye (uveal melanoma). In very rare cases melanoma can also happen in the lung which is known as primary pulmonary melanoma and only happens in 0.01% of primary lung tumors. In women, melanomas most commonly occur on the legs; while in men, on the back. Melanoma is frequently referred to as malignant melanoma. However, the medical community stresses that there is no such thing as a 'benign melanoma' and recommends that the term 'malignant melanoma' should be avoided as redundant. About 25% of melanomas develop from nevus, moles. Changes in a mole that can indicate melanoma include increaseespecially rapid increasein size, irregular edges, change in color, itchiness, or nevus#Classification, skin breakdown. The primary cause of melanoma is ultraviolet light (UV) exposure in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iovance Biotherapeutics
Iovance Biotherapeutics, Inc. is a biopharmaceutical startup based in San Carlos, California. The company works to develop tumor-infiltrating lymphocyte (TIL) therapies against cancer. History The company was founded in 2007 as Genesis Biopharma. In 2013, Lion merged with Genesis Biopharma аnd became lion bio Pharma, then rebranded to Iovance in 2017. In 2024 the US FDA gave accelerated approval to Lifileucel, the company's tumor-infiltrating lymphocyte drug for the treatment of unresectable or metastatic melanoma. See also *Kite Pharma Kite Pharma, Inc. is an American biotechnology company that develops cancer immunotherapy products with a primary focus on genetically engineered autologous CAR T cell therapy - a cell-based therapy which relies on chimeric antigen receptors a ... References External links * {{med-company-stub Biotechnology companies of the United States Pharmaceutical companies established in 2007 Biotechnology companies established in 2013 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water per os, by mouth. It may also be used to administer pharmaceutical drug, medications or other medical therapy such as blood transfusion, blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-KIT
Proto-oncogene c-KIT is the gene encoding the receptor tyrosine kinase protein known as tyrosine-protein kinase KIT, CD117 (cluster of differentiation 117) or mast/stem cell growth factor receptor (SCFR). Multiple transcript variants encoding different isoforms have been found for this gene. KIT was first described by the German biochemist Axel Ullrich in 1987 as the cellular homolog of the feline sarcoma viral oncogene v-kit. Function KIT is a cytokine receptor expressed on the surface of hematopoietic stem cells as well as other cell types. Altered forms of this receptor may be associated with some types of cancer. KIT is a receptor tyrosine kinase type III, which binds to stem cell factor, also known as "steel factor" or "c-kit ligand". When this receptor binds to stem cell factor (SCF) it forms a dimer that activates its intrinsic tyrosine kinase activity, that in turn phosphorylates and activates signal transduction molecules that propagate the signal in the cell. After a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer Treatments
Cancer treatments are a wide range of treatments available for the many different types of cancer, with each cancer type needing its own specific treatment. Treatments can include surgery, chemotherapy, radiation therapy, hormonal therapy, targeted therapy including small-molecule drugs or monoclonal antibodies, and PARP inhibitors such as olaparib. Other therapies include hyperthermia, immunotherapy, photodynamic therapy, and stem-cell therapy. Most commonly cancer treatment involves a series of separate therapies such as chemotherapy before surgery. Angiogenesis inhibitors are sometimes used to enhance the effects of immunotherapies. The choice of therapy depends upon the location and grade of the tumor and the stage of the disease, as well as the general state of the patient. Biomarker testing can help to determine the type of cancer, and indicate the best therapy. A number of experimental cancer treatments are continuously under development. In 2023 it was estimated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Priority Review
Priority review is a program of the United States Food and Drug Administration (FDA) to expedite the review process for drugs that are expected to have a particularly great impact on the treatment of a disease. The priority review voucher program is a program that grants a voucher for priority review to a drug developer as an incentive to develop treatments for disease indications with limited profitability. Priority review vouchers are currently earned by pharmaceutical companies for the development and approval of drugs treating neglected tropical diseases, rare pediatric diseases, and "medical countermeasures" for terrorism. The voucher can be used for future drugs that could have wider indications for use, but the company is required to pay a fee (approximately $2.8 million) to use the voucher. When seeking approval for a drug, manufacturers can apply to the FDA for priority review. This is granted when a drug is intended to treat a serious condition and would "provide a sig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast track is one of five FDA approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and regenerative medicine advanced therapy. Fast track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical need. Serious condition: det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a medication, pharmaceutical agent that is developed to treat certain rare medical conditions. An orphan drug would not be profitable to produce without government assistance, due to the small population of patients affected by the conditions. The conditions that orphan drugs are used to treat are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy that depends on the legislation (if there is any) of the country. Designation of a drug as an orphan drug has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug medical research, research and development. Examples of this can be that in the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Endpoint
Clinical endpoints or clinical outcomes are outcome measures referring to occurrence of disease, symptom, sign or laboratory abnormality constituting a target outcome in clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sense, a clinical endpoint is included in the entities of interest in a trial. The re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |