|
Law Of Dilution
Wilhelm Ostwald’s dilution law is a relationship proposed in 1888 between the dissociation constant ' and the degree of dissociation ' of a weak electrolyte. The law takes the form :K_d = \cfrac = \frac \cdot c_0 Where the square brackets denote concentration, and is the total concentration of electrolyte. Using \alpha=\Lambda_c/\Lambda_0, where \Lambda_c is the molar conductivity at concentration c and \Lambda_0 is the limiting value of molar conductivity extrapolated to zero concentration or infinite dilution, this results in the following relation: :K_d = \cfrac \cdot c_0 Derivation Consider a binary electrolyte AB which dissociates reversibly into A+ and B− ions. Ostwald noted that the law of mass action can be applied to such systems as dissociating electrolytes. The equilibrium state is represented by the equation: :AB + B^- If ' is the fraction of dissociated electrolyte, then ' is the concentration of each ionic species. must, therefore be the fraction o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wilhelm Ostwald
Friedrich Wilhelm Ostwald (; 4 April 1932) was a Baltic German chemist and philosopher. Ostwald is credited with being one of the founders of the field of physical chemistry, with Jacobus Henricus van 't Hoff, Walther Nernst, and Svante Arrhenius. He received the Nobel Prize in Chemistry in 1909 for his scientific contributions to the fields of catalysis, chemical equilibria and reaction velocities. Following his 1906 retirement from academic life, Ostwald became much involved in philosophy, art, and politics. He made significant contributions to each of these fields. He has been described as a polymath. Early life and education Ostwald was born ethnically Baltic German in Riga, Russian Empire (now Latvia) to master-cooper Gottfried Wilhelm Ostwald (1824–1903) and Elisabeth Leuckel (1824–1903). He was the middle child of three, born after Eugen (1851–1932) and before Gottfried (1855–1918). Ostwald developed an interest in science as a child and conducted experiment ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
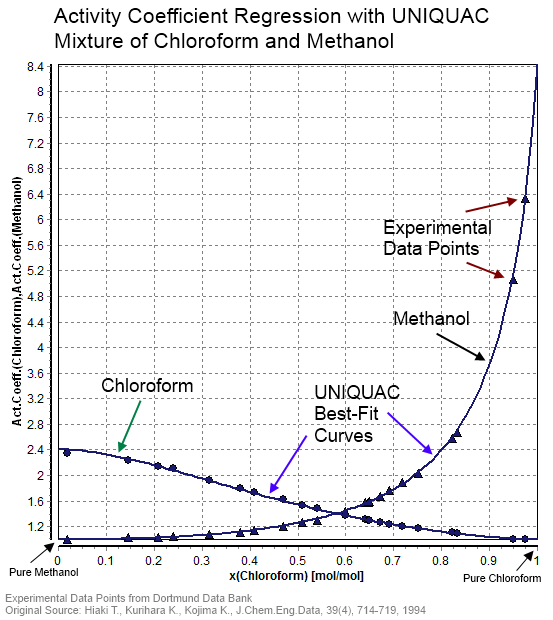
Thermodynamic Activity
In chemical thermodynamics, activity (symbol ) is a measure of the "effective concentration" of a species in a mixture, in the sense that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution. The term "activity" in this sense was coined by the American chemist Gilbert N. Lewis in 1907. By convention, activity is treated as a dimensionless quantity, although its value depends on customary choices of standard state for the species. The activity of pure substances in condensed phases (solid or liquids) is normally taken as unity (the number 1). Activity depends on temperature, pressure and composition of the mixture, among other things. For gases, the activity is the effective partial pressure, and is usually referred to as fugacity. The difference between activity and other measures of concentration arises because the interactions between different types of molecules in non-ideal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molar Conductivity
The molar conductivity of an electrolyte solution is defined as its conductivity divided by its molar concentration. : \Lambda_\text = \frac, where: : ''κ'' is the measured conductivity (formerly known as specific conductance), : ''c'' is the molar concentration of the electrolyte. The SI unit of molar conductivity is siemens metres squared per mole (S m2 mol−1). However, values are often quoted in S cm2 mol−1. In these last units, the value of Λm may be understood as the conductance of a volume of solution between parallel plate electrodes one centimeter apart and of sufficient area so that the solution contains exactly one mole of electrolyte. Variation of molar conductivity with dilution There are two types of electrolytes: strong and weak. Strong electrolytes usually undergo complete ionization, and therefore they have higher conductivity than weak electrolytes, which undergo only partial ionization. For strong electrolytes, such as salts, stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Association
In chemistry, ion association is a chemical reaction whereby ions of opposite electric charge come together in solution to form a distinct chemical entity. Ion associates are classified, according to the number of ions that associate with each other, as ion pairs, ion triplets, etc. Ion pairs are also classified according to the nature of the interaction as contact, solvent-shared or solvent-separated. The most important factor to determine the extent of ion association is the dielectric constant of the solvent. Ion associates have been characterized by means of vibrational spectroscopy, as introduced by Niels Bjerrum, and dielectric-loss spectroscopy. Classification of ion pairs ''Ion pairs'' are formed when a cation and anion, which are present in a solution of an ionizable substance, come together to form a discrete chemical species. There are three distinct types of ''ion pairs'', depending on the extent of solvation of the two ions. For example, magnesium sulphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Transport Number
In chemistry, ion transport number, also called the transference number, is the fraction of the total electric current carried in an electrolyte by a given ionic species : :t_i = \frac Differences in transport number arise from differences in electrical mobility. For example, in an aqueous solution of sodium chloride, less than half of the current is carried by the positively charged sodium ions (cations) and more than half is carried by the negatively charged chloride ions (anions) because the chloride ions are able to move faster, i.e., chloride ions have higher mobility than sodium ions. The sum of the transport numbers for all of the ions in solution always equals unity: :\sum_i t_i = 1 The concept and measurement of transport number were introduced by Johann Wilhelm Hittorf in the year 1853. Liquid junction potential can arise from ions in a solution having different ion transport numbers. At zero concentration, the limiting ion transport numbers may be expressed in ter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by :\mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Coefficient
An osmotic coefficient \phi is a quantity which characterises the deviation of a solvent from ideal behaviour, referenced to Raoult's law. It can be also applied to solutes. Its definition depends on the ways of expressing chemical composition of mixtures. The osmotic coefficient based on molality ''m'' is defined by: \phi = \frac and on a mole fraction basis by: \phi = -\frac where \mu_A^* is the chemical potential of the pure solvent and \mu_A is the chemical potential of the solvent in a solution, ''M''A is its molar mass, ''x''A its mole fraction, ''R'' the gas constant and ''T'' the temperature in Kelvin. The latter osmotic coefficient is sometimes called the rational osmotic coefficient. The values for the two definitions are different, but since \ln x_A = - \ln \left(1 + M_A \sum_i m_i \right) \approx - M_A \sum_i m_i, the two definitions are similar, and in fact both approach 1 as the concentration goes to zero. Applications For liquid solutions, the osmotic coeff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autosolvolysis
In chemistry, molecular autoionization (or self-ionization) is a chemical reaction between molecules of the same substance to produce ions. If a pure liquid partially dissociates into ions, it is said to be self-ionizing. In most cases the oxidation number on all atoms in such a reaction remains unchanged. Such autoionization can be '' protic'' ( transfer), or ''non-protic''. Examples Protic solvents Protic solvents often undergo some autoionization (in this case autoprotolysis): *2 H2O H3O+ + OH- **The self-ionization of water is particularly well studied, due to its implications for acid-base chemistry of aqueous solutions. *2 NH3 NH4+ + NH2- *2 H2SO4 H3SO4+ + HSO4- *3 HF H2F+ + HF2- **Here proton transfer between two HF combines with homoassociation of and a third HF to form Non-protic solvents *2 PF5 PF6- + PF4+ *N2O4 NO+ + NO3- ** Here the nitrogen oxidation numbers change from (+4 and +4) to (+3 and +5). *2 BrF3 BrF2+ + BrF4- These solvents all poss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye–Hückel Theory
The Debye–Hückel theory was proposed by Peter Debye and Erich Hückel as a theoretical explanation for departures from ideality in solutions of electrolytes and plasmas. It is a linearized Poisson–Boltzmann model, which assumes an extremely simplified model of electrolyte solution but nevertheless gave accurate predictions of mean activity coefficients for ions in dilute solution. The Debye–Hückel equation provides a starting point for modern treatments of non-ideality of electrolyte solutions. Overview In the chemistry of electrolyte solutions, an ideal solution is a solution whose colligative properties are proportional to the concentration of the solute. Real solutions may show departures from this kind of ideality. In order to accommodate these effects in the thermodynamics of solutions, the concept of activity was introduced: the properties are then proportional to the activities of the ions. Activity, ''a'', is proportional to concentration, ''c''. The proporti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by :\mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Thermodynamics
Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the ''spontaneity'' of processes. The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the "fundamental equations of Gibbs" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics. History In 1865, the German physicist Rudolf Clausius, in his ''Mechanical Theory of Heat'', suggested that the principles of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociation Constant
In chemistry, biochemistry, and pharmacology, a dissociation constant (K_D) is a specific type of equilibrium constant that measures the propensity of a larger object to separate (dissociate) reversibly into smaller components, as when a complex falls apart into its component molecules, or when a salt splits up into its component ions. The dissociation constant is the inverse of the association constant. In the special case of salts, the dissociation constant can also be called an ionization constant. For a general reaction: : A_\mathit B_\mathit \mathit A + \mathit B in which a complex \ce_x \ce_y breaks down into ''x'' A subunits and ''y'' B subunits, the dissociation constant is defined as : K_D = \frac where and ''x'' B''y''are the equilibrium concentrations of A, B, and the complex A''x'' B''y'', respectively. One reason for the popularity of the dissociation constant in biochemistry and pharmacology is that in the frequently en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |