|
Langlois Reagent
Sodium trifluoromethanesulfinate (CF3SO2Na) is the sodium salt of trifluoromethanesulfinic acid. Together with t-butyl hydroperoxide, an oxidant, this compound was found to be a suitable reagent for introducing trifluoromethyl groups onto electron-rich aromatic compounds by Langlois; this reagent is also known as the Langlois reagent. This reaction operates via a free radical mechanism. This reagent is also able to trifluoromethylate electron-deficient aromatic compounds under biphasic conditions. Zinc difluoromethanesulfinate, a related polymeric coordination complex A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ..., is able to introduce difluoromethyl groups (CHF2-) onto aromatic compounds under similar biphasic conditions as well. With the use of DMSO as an oxidant, it provides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T-butyl Hydroperoxide
''tert''-Butyl hydroperoxide (tBuOOH) is the organic compound with the formula (CH3)3COOH. It is one of the most widely used hydroperoxides in a variety of oxidation processes, for example the Halcon process. It is normally supplied as a 69–70% aqueous solution. Compared to hydrogen peroxide and organic peracids, ''tert''-butyl hydroperoxide is less reactive and more soluble in organic solvents. Overall, it is renowned for the convenient handling properties of its solutions. Its solutions in organic solvents are highly stable. Application Industrially, ''tert''-butyl hydroperoxide is used to prepare propylene oxide. In the Halcon process, molybdenum-based catalysts are used for this reaction: :(CH3)3COOH + CH2=CHCH3 → (CH3)3COH + CH2OCHCH3 The byproduct t-butanol, which can be dehydrated to isobutene and converted to MTBE. On a much smaller scale, ''tert''-butyl hydroperoxide is used to produce some fine chemicals by the Sharpless epoxidation. Synthesis and product ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
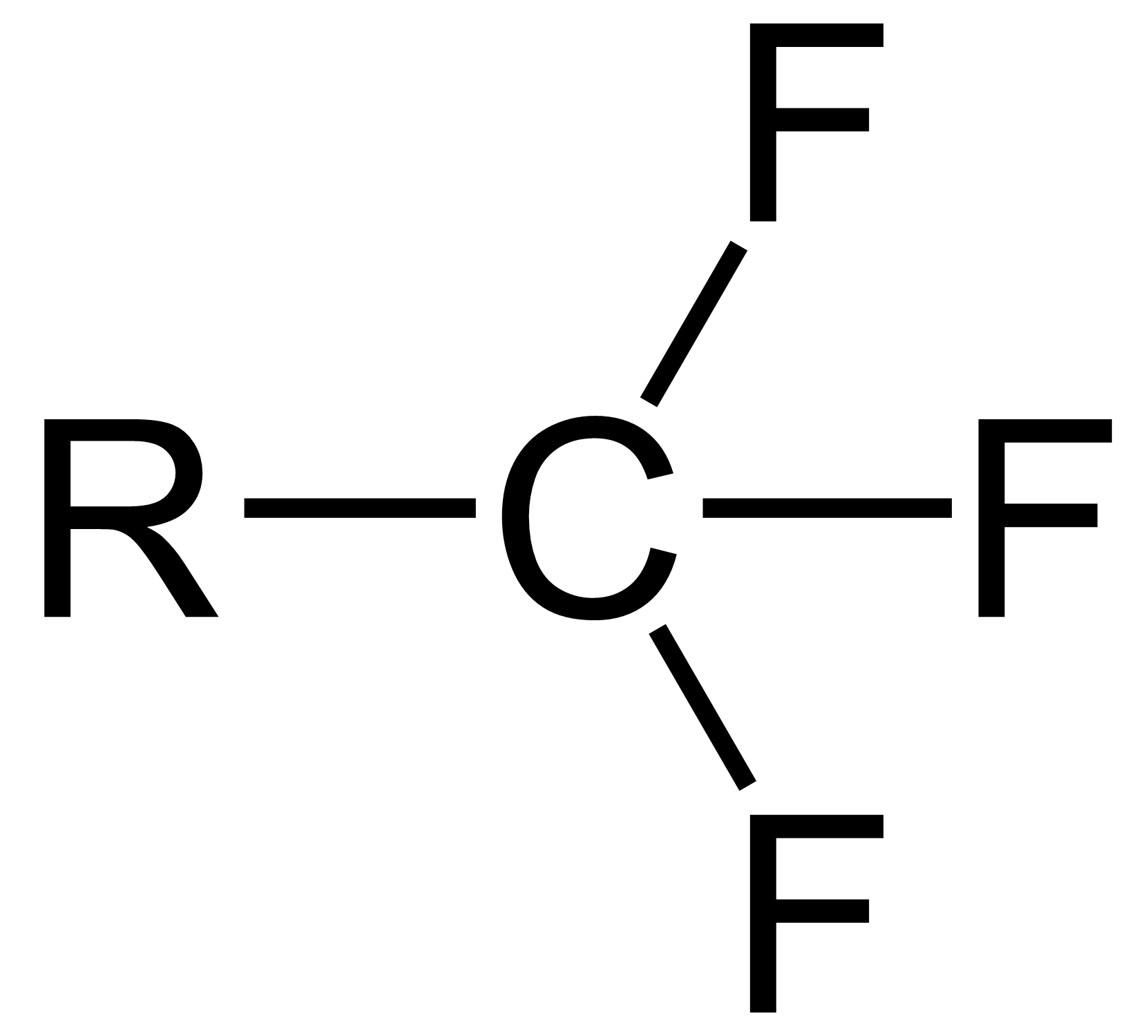
Trifluoromethyl Group
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing. Ageing Ailments of unknown cause Biogerontology Biological processes Causes of death Cellular processes Gerontology Life extension Metabolic disorders Metabolism Old age Time in life Wikipedia categories named after diseases and disorders {{CatAutoTOC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Difluoromethanesulfinate
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity (electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Compounds
Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. As a result, sodium usually forms ionic compounds involving the Na+ cation. Sodium is a reactive alkali metal and is much more stable in ionic compounds. It can also form intermetallic compounds and organosodium compounds. Sodium compounds are often soluble in water. Metallic sodium Metallic sodium is generally less reactive than potassium and more reactive than lithium. Sodium metal is highly reducing, with the standard reduction potential for the Na+/Na couple being −2.71 volts, though potassium and lithium have even more negative potentials. The thermal, fluidic, chemical, and nuclear properties of molten sodium metal have caused it to be one of the main coolants of choice for the fast breeder reactor. Such nuclear reactors are seen as a crucial step for the production of clean energy. Salts and oxides Sodium compounds are of immense commercial importance, being particularly centra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine Compounds
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding (a weaker bridging link to certain nonmetals). Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds.In this article, metalloids are not treated separately from metals and nonmetals, but among elements they are closer to. For example, germanium is treated as a metal, and silicon as a nonmetal. Antimony is included for comparison among nonmetals, even though it is closer to metals chemically than to nonmetals. The no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagents For Organic Chemistry
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |