|
LAMP1
Lysosomal-associated membrane protein 1 (LAMP-1) also known as lysosome-associated membrane glycoprotein 1 and CD107a (Cluster of Differentiation 107a), is a protein that in humans is encoded by the ''LAMP1'' gene. The human ''LAMP1'' gene is located on the long arm (q) of chromosome 13 at region 3, band 4 (13q34). Lysosomal-associated membrane protein 1 is a glycoprotein from a family of Lysosome-associated membrane glycoproteins. The LAMP-1 glycoprotein is a type I transmembrane protein which is expressed at high or medium levels in at least 76 different normal tissue cell types. It resides primarily across l ysosomal membranes, and functions to provide selectins with carbohydrate ligands. CD107a has also been shown to be a marker of degranulation on lymphocytes such as CD8+ and NK cells, and may also play a role in tumor cell differentiation and metastasis. Structure Residing primarily across lysosomal membranes, these glycoproteins consist of a large, highly glycosylated e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LAMP2
Lysosome-associated membrane protein 2 (LAMP2), also known as CD107b (Cluster of Differentiation 107b) and Mac-3, is a human gene. Its protein, LAMP2, is one of the lysosome-associated membrane glycoproteins. The protein encoded by this gene is a member of a family of membrane glycoproteins. This glycoprotein provides selectins with carbohydrate ligands. It may play a role in tumor cell metastasis. It may also function in the protection, maintenance, and adhesion of the lysosome. Alternative splicing of the gene produces three variants - LAMP-2A, LAMP-2B and LAMP-2C. LAMP-2A is the receptor for chaperone-mediated autophagy. Recently it has been determined that antibodies against LAMP-2 account for a fraction of patients who get a serious kidney disease termed focal necrotizing glomerulonephritis. LAMP-2B is associated with Danon disease. Structure and tissue distribution The gene for LAMP2 has 9 coding exons and 2 alternate last exons, 9a and 9b. When the last exon is spli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysosome-associated Membrane Glycoproteins
Lysosome-associated membrane glycoproteins (LAMPs) are integral membrane proteins, specific to lysosomes, and whose exact biological function is not yet clear. Structurally, the lamp proteins consist of two internally homologous lysosome-luminal domains separated by a proline-rich hinge region; at the C-terminal extremity there is a transmembrane region (TM) followed by a very short cytoplasmic tail (C). In each of the duplicated domains, there are two conserved disulfide bonds. This structure is schematically represented in the figure below. +-----+ +-----+ +-----+ +-----+ , , , , , , , , +--------------------------++Hinge++--------------------------++TM++C+ In mammals, there are two closely related types of lamp: LAMP1 and LAMP2. CD69 (also called gp110 or macrosialin) is a heavily glycosylated integral membrane protein whose structure consists of a mucin-like domain followed by a pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Five clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenotype
In genetics, the phenotype () is the set of observable characteristics or traits of an organism. The term covers the organism's morphology or physical form and structure, its developmental processes, its biochemical and physiological properties, its behavior, and the products of behavior. An organism's phenotype results from two basic factors: the expression of an organism's genetic code, or its genotype, and the influence of environmental factors. Both factors may interact, further affecting phenotype. When two or more clearly different phenotypes exist in the same population of a species, the species is called polymorphic. A well-documented example of polymorphism is Labrador Retriever coloring; while the coat color depends on many genes, it is clearly seen in the environment as yellow, black, and brown. Richard Dawkins in 1978 and then again in his 1982 book ''The Extended Phenotype'' suggested that one can regard bird nests and other built structures such as cad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, nucleic acids, and proteins) into smaller units (such as monosaccharides, fatty acids, nucleotides, and amino acids, respectively). Catabolism is the breaking-down aspect of metabolism, whereas anabolism is the building-up aspect. Cells use the monomers released from breaking down polymers to either construct new polymer molecules or degrade the monomers further to simple waste products, releasing energy. Cellular wastes include lactic acid, acetic acid, carbon dioxide, ammonia, and urea. The formation of these wastes is usually an oxidation process involving a release of chemical free energy, some of which is lost as heat, but the rest of which is used to drive the synthesis of adenosine triphosphate (ATP). This molecule acts as a way f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan (or, to be more specific, a glucan) composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked ''N''-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched. Glycans and proteins Glycans can be found attached to proteins as in glycoproteins and proteoglycans. In general, they are found on the exterior surface of cells. O- and N-linked glycans are very common in eukaryotes but may also be found, although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cisterna
A cisterna (plural cisternae) is a flattened membrane vesicle found in the endoplasmic reticulum and Golgi apparatus. Cisternae are an integral part of the packaging and modification processes of proteins occurring in the Golgi. Function Proteins begin on the cis side of the Golgi (the side facing the ER) and exit on the trans side (the side facing the plasma membrane). Throughout their journey in the cisterna, the proteins are packaged and are modified for transport throughout the cell. The number of cisterna in the Golgi stack is dependent on the organism and cell type. The structure, composition, and function of each of the cisternae may be different inside the Golgi stack. These different variations of Golgi cisternae are categorized into 3 groups; cis Golgi network, medial, and trans Golgi network. The cis Golgi network is the first step in the cisternal structure of a protein being packaged, while the trans Golgi network is the last step in the cisternal structure when the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
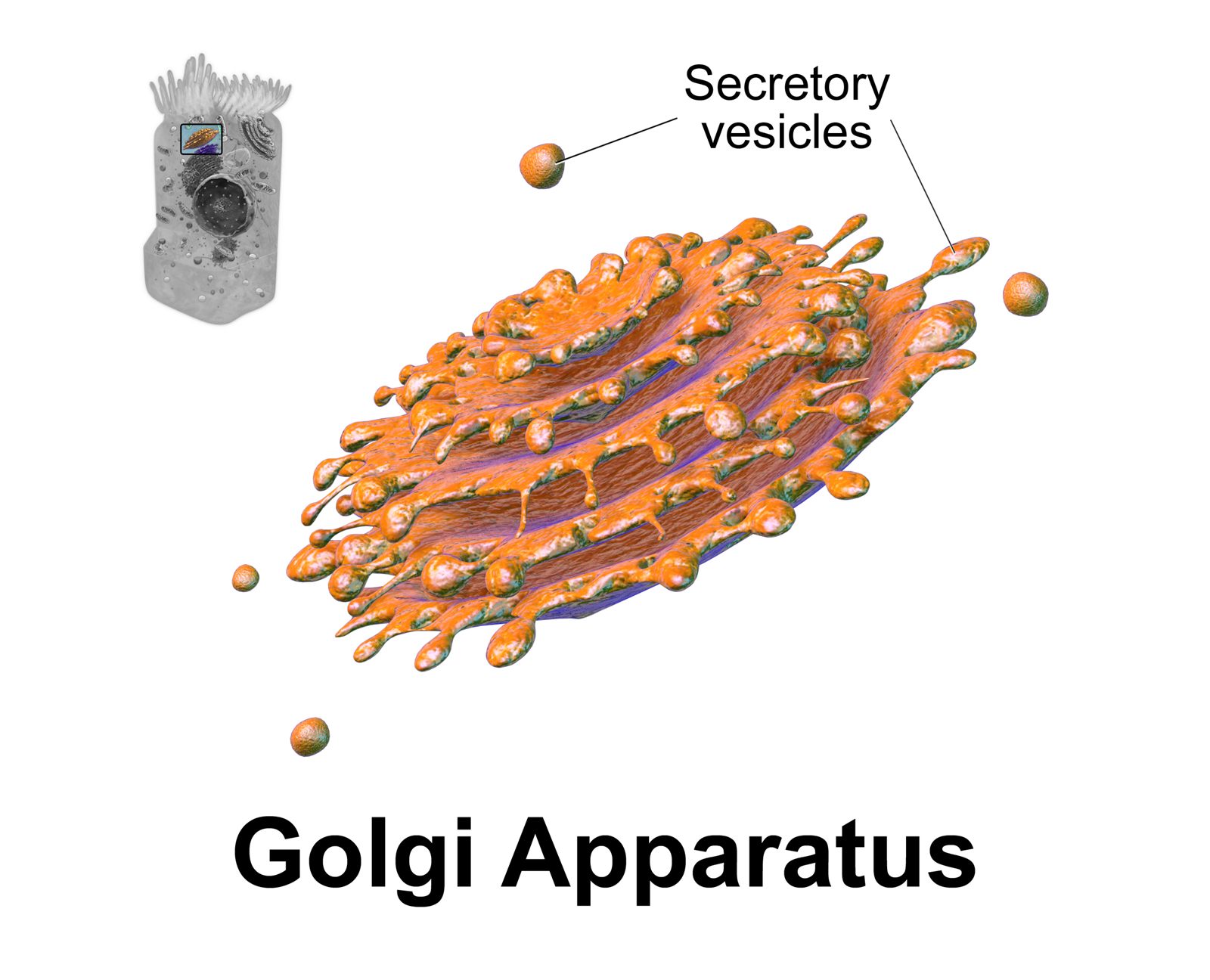
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sialic Acid
Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone. The term "sialic acid" (from the Greek for saliva, - ''síalon'') was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is ''N''-acetylneuraminic acid (Neu5Ac or NANA) found in animals and some prokaryotes. Sialic acids are found widely distributed in animal tissues and related forms are found to a lesser extent in other organisms like in some micro-algae, bacteria and archaea. Sialic acids are commonly part of glycoproteins, glycolipids or gangliosides, where they decorate the end of sugar chains at the surface of cells or soluble proteins. However, sialic acids have been also observed in ''Drosophila'' embryos and other insects. Generally, plants seem not to contain or display sialic acids. In humans the brain has the highest sialic acid content, where these acids play an important role in neural transmission and ganglioside structure in synaptogene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-glycosylation
''N''-linked glycosylation, is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom (the amide nitrogen of an asparagine (Asn) residue of a protein), in a process called ''N''-glycosylation, studied in biochemistry. This type of linkage is important for both the structure and function of many eukaryotic proteins. The ''N''-linked glycosylation process occurs in eukaryotes and widely in archaea, but very rarely in bacteria. The nature of ''N''-linked glycans attached to a glycoprotein is determined by the protein and the cell in which it is expressed. It also varies across species. Different species synthesize different types of ''N''-linked glycan. Energetics of bond formation There are two types of bonds involved in a glycoprotein: bonds between the saccharides residues in the glycan and the linkage between the glycan chain and the protein molecule. The sugar moieties are linked t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |