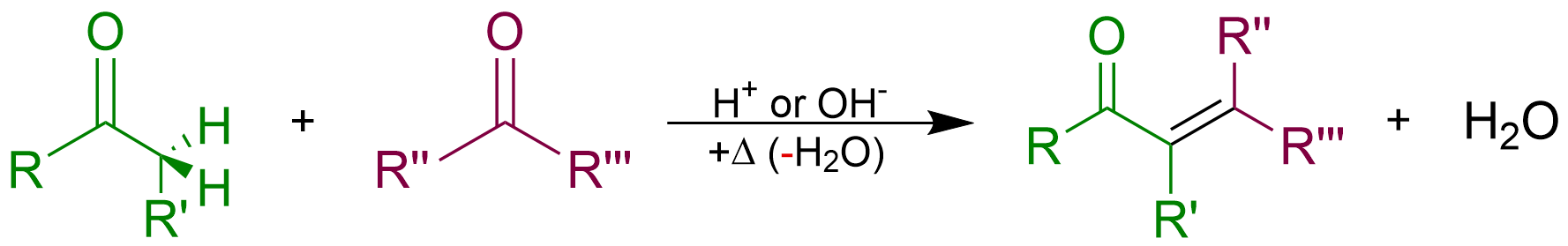|
Kostanecki Acylation
The Kostanecki acylation is a method used in organic synthesis to form chromones or coumarins by acylation of ''O''-hydroxyaryl ketones with aliphatic acid anhydrides, followed by cyclization. If benzoic anhydride (or benzoyl chloride) is used, a particular type of chromone called a flavone is obtained. Mechanism The mechanism consists of three well-differentiated reactions:Ellis, G. P. (1977) ''Chromenes, Chromanones, and Chromones from The Chemistry of Heterocyclic Compounds'', Weissberger, A. and Taylor, E. C., eds.; Wiley & Sons: New York, vol. 31, p. 495. # Phenol ''O''-acylation with formation of a tetrahedral intermediate # Intramolecular aldol condensation to cyclize and to form a hydroxydihydrochromone # Elimination of the hydroxyl group to form the chromone (or coumarin) Examples * Alvocidib (flavopiridol) * Dimefline *Flavoxate See also * Allan–Robinson reaction * Baker–Venkataraman rearrangement The Baker–Venkataraman rearrangement is the chemical reaction o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Name Reactions
A name reaction is a chemical reaction named after its discoverers or developers. Among the tens of thousands of organic reactions that are known, hundreds of such reactions are well-known enough to be named after people. Well-known examples include the Grignard reaction, the Sabatier reaction, the Wittig reaction, the Claisen condensation, the Friedel-Crafts acylation, and the Diels-Alder reaction. Books have been published devoted exclusively to name reactions;Alfred Hassner, C. Stumer. ''Organic syntheses based on name reactions''. Elsevier, 2002. Li, Jie Jack. ''Name Reactions: A Collection of Detailed Reaction Mechanisms''. Springer, 2003. the Merck Index, a chemical encyclopedia, also includes an appendix on name reactions. As organic chemistry developed during the 20th century, chemists started associating synthetically useful reactions with the names of the discoverers or developers; in many cases, the name is merely a mnemonic. Some cases of reactions that were not reall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baker–Venkataraman Rearrangement
The Baker–Venkataraman rearrangement is the chemical reaction of 2-acetoxyacetophenones with base to form 1,3-diketones. This rearrangement reaction proceeds via enolate formation followed by acyl transfer. It is named after the scientists Wilson Baker and K. Venkataraman. The Baker–Venkataraman rearrangement is often used in the synthesis of chromones and flavones. ''(also in thCollective Volume (1963) 4: 478 (PDF).'' After the base-catalyzed rearrangement, treatment with acid generally affords the chromone or flavone core, though other milder methods have been reported. Mechanism A base abstracts the hydrogen atom alpha to the aromatic ketone, forming an enolate. Then, the enolate attacks the ester carbonyl to form a cyclic alkoxide. The cyclic intermediate is opened up to form a more stable phenolate, which is protonated during acidic work-up to give the desired product. To complete the construction of the chromone or flavone core, cyclodehydration is required. This ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allan–Robinson Reaction
The Allan–Robinson reaction is the chemical reaction of o-hydroxyaryl ketones with aromatic anhydrides to form flavones (or isoflavones). If aliphatic anhydrides are used, coumarins can also be formed. (See Kostanecki acylation.) : Mechanism : The first step is an enolization which is a proton transfer created a hydroxide instead of a carbonyl and an alkene instead of an alkane. The second step is an acylation in which the newly formed bond from the enolization attacks an electrophilic carbon in the anhydride. The third step displays the carboxylate functionality leaving since it is the best leaving group. As a result, the resulting carboxylate attacks an alpha hydrogen to create the enol functionality again in step four. The fifth step shows the nucleophilic hydroxide attacking the carbonyl carbon to create a new six membered heterocyclic ring. The resulting structure undergoes a proton transfer in step 6 to achieve the final product. All six of these steps occur in the reac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavoxate
Flavoxate is an anticholinergic with antimuscarinic effects. Its muscle relaxant properties may be due to a direct action on the smooth muscle rather than by antagonizing muscarinic receptors. Clinical uses Flavoxate is used to treat urinary bladder spasms. It is available under the trade name Urispas (Paladin)(india), Genurin (by Recordati, Italy) in Italy and KSA, Uritac by El Saad company in Syria, under the name Bladderon by Nippon Shinyaku of Japan, or Bladuril in Chile, Utispas ( Apex Pharma) in Nepal. Flavoxate is indicated for symptomatic relief of interstitial cystitis Interstitial cystitis (IC), a type of bladder pain syndrome (BPS), is chronic pain in the bladder and pelvic floor of unknown cause. It is the urologic chronic pelvic pain syndrome of women. Symptoms include feeling the need to urinate right aw ..., dysuria, urgency, nocturia, suprapubic pain, frequency and incontinence as may occur in cystitis, prostatitis, urethritis, urethrocystitis/urethrotrigo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alvocidib
Alvocidib (INN; also known as flavopiridol) is a flavonoid alkaloid CDK9 kinase inhibitor under clinical development by Tolero Pharmaceuticals for the treatment of acute myeloid leukemia. It has been studied also for the treatment of arthritis and atherosclerotic plaque formation. The target of alvocidib is the positive transcription elongation factor P-TEFb. Treatment of cells with alvocidib leads to inhibition of P-TEFb and the loss of mRNA production. The compound is a synthetic analog of natural product rohitukine which was initially extracted from ''Aphanamixis polystachya'' (formerly ''Amoora rohituka'', hence the name) and later from ''Dysoxylum binectariferum''. Orphan drug The FDA has granted orphan drug An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of ... designation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack this f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromone
Chromone (or 1,4-benzopyrone) is a derivative of benzopyran with a substituted keto group on the pyran ring. It is an isomer of coumarin. Derivatives of chromone are collectively known as ''chromones''. Most, though not all, chromones are also phenylpropanoids. Examples * 6,7-dimethoxy-2,3-dihydrochromone has been isolated from ''Sarcolobus globosus''. * Eucryphin, a chromone rhamnoside, can be isolated from the bark of ''Eucryphia cordifolia''. * Cromolyn (disodium cromoglicate) was found to inhibit antigen challenge as well as stress induced symptoms.HOWELL, J.B. & ALTOUNYAN, R.E. (1967). A double-blind trial of disodium cromoglycate in the treatment of allergic bronchial asthma. Lancet, 2, 539–542Abstract/ref> Cromoglicate is used as a mast cell stabilizer in allergic rhinitis, asthma and allergic conjunctivitis. * Nedocromil sodium was found to have a somewhat longer half-life than cromolyn; however, production was discontinued in the US in 2008. * Xanthone with a se ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kostanecki Acylation
The Kostanecki acylation is a method used in organic synthesis to form chromones or coumarins by acylation of ''O''-hydroxyaryl ketones with aliphatic acid anhydrides, followed by cyclization. If benzoic anhydride (or benzoyl chloride) is used, a particular type of chromone called a flavone is obtained. Mechanism The mechanism consists of three well-differentiated reactions:Ellis, G. P. (1977) ''Chromenes, Chromanones, and Chromones from The Chemistry of Heterocyclic Compounds'', Weissberger, A. and Taylor, E. C., eds.; Wiley & Sons: New York, vol. 31, p. 495. # Phenol ''O''-acylation with formation of a tetrahedral intermediate # Intramolecular aldol condensation to cyclize and to form a hydroxydihydrochromone # Elimination of the hydroxyl group to form the chromone (or coumarin) Examples * Alvocidib (flavopiridol) * Dimefline *Flavoxate See also * Allan–Robinson reaction * Baker–Venkataraman rearrangement The Baker–Venkataraman rearrangement is the chemical reaction o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |