|
Keith J. Laidler
Keith James Laidler (January 3, 1916 – August 26, 2003), born in England, was notable as a pioneer in chemical kinetics and authority on the physical chemistry of enzymes. Education Laidler received his early education at Liverpool College. He received his BA (1934) and MA (1938) degrees from Trinity College, Oxford University.Keith James Laidler, Physical Chemistry, A pioneer in the field of chemical kinetics and activated-complex theory The science.ca team, GCS Research Society (2001 and 2015).Keith J. Laidler (1916–20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liverpool
Liverpool is a city and metropolitan borough in Merseyside, England. With a population of in 2019, it is the 10th largest English district by population and its metropolitan area is the fifth largest in the United Kingdom, with a population of 2.24 million. On the eastern side of the Mersey Estuary, Liverpool historically lay within the ancient hundred of West Derby in the county of Lancashire. It became a borough in 1207, a city in 1880, and a county borough independent of the newly-created Lancashire County Council in 1889. Its growth as a major port was paralleled by the expansion of the city throughout the Industrial Revolution. Along with general cargo, freight, and raw materials such as coal and cotton, merchants were involved in the slave trade. In the 19th century, Liverpool was a major port of departure for English and Irish emigrants to North America. It was also home to both the Cunard and White Star Lines, and was the port of registry of the ocean li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liverpool College
Liverpool College is a school in Mossley Hill, Liverpool, England. It was one of the thirteen founding members of the Headmasters' Conference (HMC). History Liverpool College was the first of many public schools founded in the Victorian Era. The foundation stone of the original building was laid on 22 October 1840 by Edward Smith-Stanley, 14th Earl of Derby K.G. (then styled the Rt. Hon. Lord Stanley MP), the first patron of the college. A group of Christian Liverpool citizens, many of whose names are now famous in the annals of the city, then began the building of a school where education might be combined with 'sound religious knowledge'. The original building in Shaw street (now apartments) is in the so-called Tudor-Gothic style. It was designed by Mr. Harvey Lonsdale Elmes, and was erected at a cost of £35,000. The college was opened on 6 January 1843 by the Right Hon. William Ewart Gladstone (afterwards four time Prime Minister of the United Kingdom) and the same ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solution (chemistry)
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactions On Surfaces
Reactions on surfaces are reactions in which at least one of the steps of the reaction mechanism is the adsorption of one or more reactants. The mechanisms for these reactions, and the rate equations are of extreme importance for heterogeneous catalysis. Via scanning tunneling microscopy, it is possible to observe reactions at the solid gas interface in real space, if the time scale of the reaction is in the correct range. Reactions at the solid–gas interface are in some cases related to catalysis. Simple decomposition If a reaction occurs through these steps: : A + S ⇌ AS → Products where A is the reactant and S is an adsorption site on the surface and the respective rate constants for the adsorption, desorption and reaction are ''k''1, ''k''−1 and ''k''2, then the global reaction rate is: :r=k_2 C_\mathrm=k_2 \theta C_\mathrm where: * ''r'' is the rate, mol·''m''−2·s−1 *C_Ais the concentration of adsorbate, ''mol·m−3'' *C_\mathrm is the surface concentrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
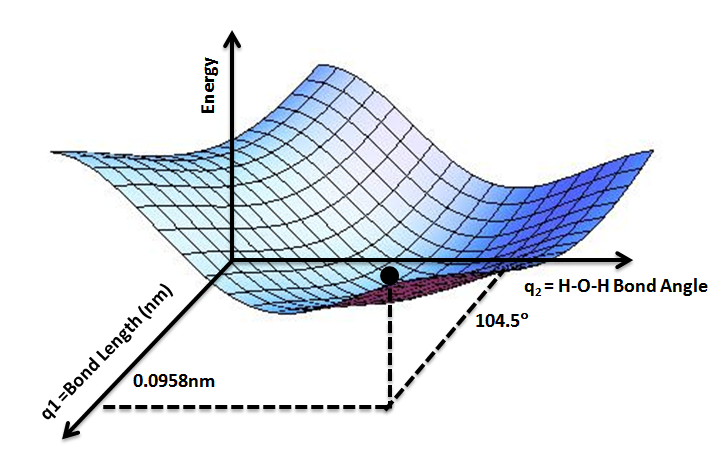
Potential Energy Surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential. It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground). The PES concept finds application in fields such as chemistry and physics, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excited State
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers to an increase in energy level above a chosen starting point, usually the ground state, but sometimes an already excited state. The temperature of a group of particles is indicative of the level of excitation (with the notable exception of systems that exhibit negative temperature). The lifetime of a system in an excited state is usually short: spontaneous or induced emission of a quantum of energy (such as a photon or a phonon) usually occurs shortly after the system is promoted to the excited state, returning the system to a state with lower energy (a less excited state or the ground state). This return to a lower energy level is often loosely described as decay and is the inverse of excitation. Long-lived excited states are often called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reactivity (chemistry)
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy. ''Reactivity'' refers to: * the chemical reactions of a single substance, * the chemical reactions of two or more substances that interact with each other, * the systematic study of sets of reactions of these two kinds, * methodology that applies to the study of reactivity of chemicals of all kinds, * experimental methods that are used to observe these processes * theories to predict and to account for these processes. The chemical reactivity of a single substance (reactant) covers its behavior in which it: * Decomposes * Forms new substances by addition of atoms from another reactant or reactants * Interacts with two or more other reactants to form two or more products The chemical reactivity of a substance can refer to the variety of circumstances (conditions that include temperature, pressure, prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition State Theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ''H''‡, also written Δ‡''H''ɵ), the standard entropy of activation (Δ''S''‡ or Δ‡''S''ɵ), and the standard Gibbs energy of activation (Δ''G''‡ or Δ‡''G''ɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest ''at the transition state''; Δ''H''‡ is the difference between the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Canada
The Royal Society of Canada (RSC; french: Société royale du Canada, SRC), also known as the Academies of Arts, Humanities and Sciences of Canada (French: ''Académies des arts, des lettres et des sciences du Canada''), is the senior national, bilingual council of distinguished Canadian scholars, humanists, scientists and artists. The primary objective of the RSC is to promote learning and research in the arts, the humanities and the sciences. The RSC is Canada's National Academy and exists to promote Canadian research and scholarly accomplishment in both official languages, to recognize academic and artistic excellence, and to advise governments, non-governmental organizations and Canadians on matters of public interest. History In the late 1870s, the Governor General of Canada, the Marquis of Lorne, determined that Canada required a cultural institution to promote national scientific research and development. Since that time, succeeding Governor Generals have remained involved w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samuel Glasstone
Samuel Glasstone (3 May 1897 – 16 November 1986) was a British-born American academic and writer of scientific books. He authored over 40 popular textbooks on physical chemistry and electrochemistry, reaction rates, nuclear weapons effects, nuclear reactor engineering, Mars, space sciences, the environmental effects of nuclear energy and nuclear testing Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected by .... Early life Glasstone was born on 3 May 1897 in London. He received two doctorates, in 1922 and 1926 (PhD and DSc), in chemistry at London University. Glasstone discovered the C–H···O interaction in 1937. After several academic appointments in England, he moved to the US in 1939 and became a naturalized citizen in 1944. Publications His book ''The Effect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry Eyring (chemist)
Henry Eyring (February 20, 1901 – December 26, 1981) was a Mexico-born United States theoretical chemist whose primary contribution was in the study of chemical reaction rates and intermediates. Eyring developed the Absolute Rate Theory or Transition state theory of chemical reactions, connecting the fields of chemistry and physics through atomic theory, quantum theory, and statistical mechanics. History Eyring, a third-generation member of the Church of Jesus Christ of Latter-day Saints (LDS Church), was reared on a cattle ranch in Colonia Juárez, Chihuahua, a Mormon colony, for the first 11 years of his life. His father, Edward Christian Eyring, practiced plural marriage; Edward married Caroline Romney (1893) and her sister Emma Romney (1903), both daughters of Miles Park Romney, the great-grandfather of Mitt Romney. In July 1912, the Eyrings and about 4,200 other immigrants were driven out of Mexico by violent insurgents during the Mexican Revolution and moved to El Paso, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |