|
Kopp's Law
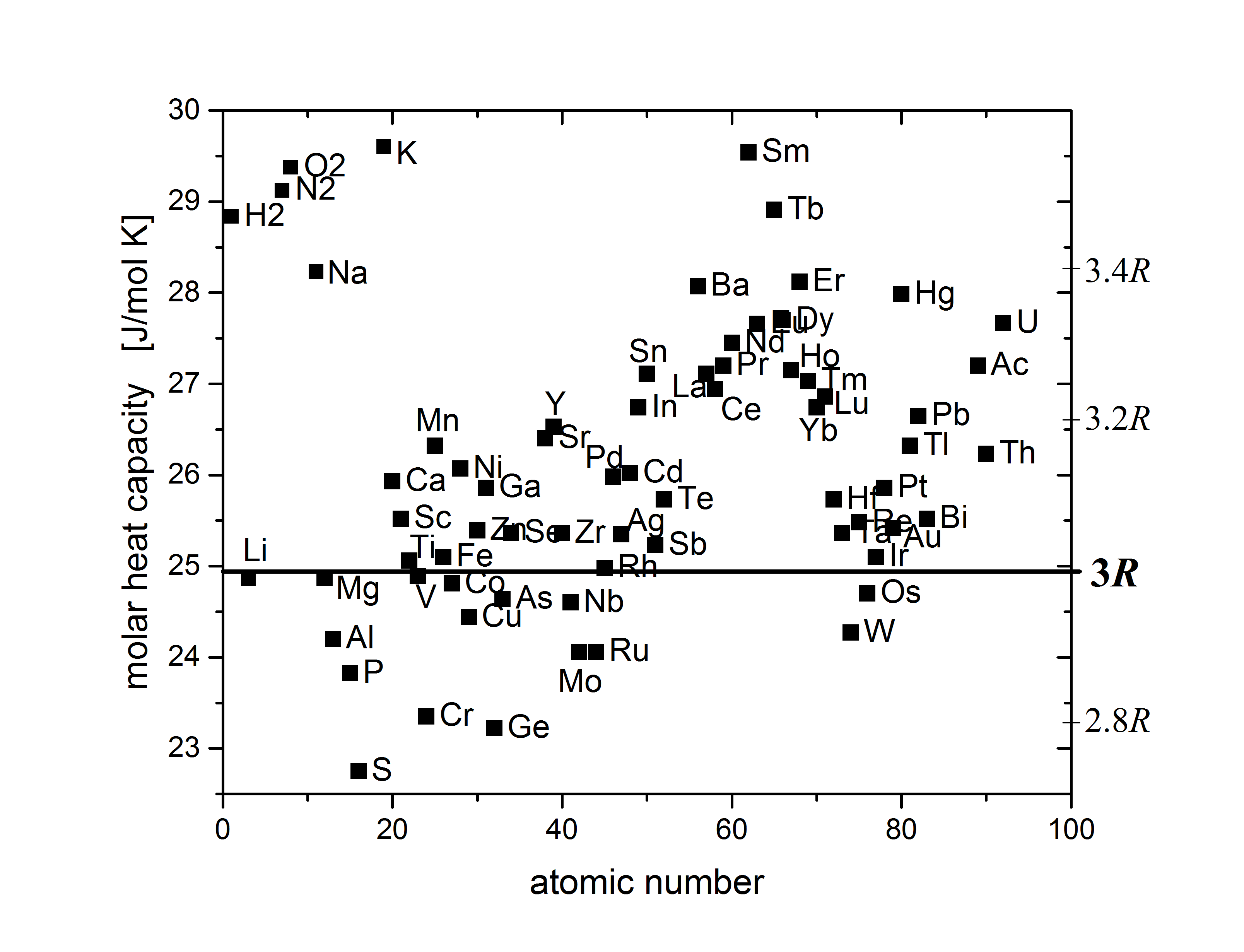
Kopp's law can refer to either of two relationships discovered by the German chemist Hermann Franz Moritz Kopp (1817–1892). #Kopp found "that the molecular heat capacity of a solid compound is the sum of the atomic heat capacities of the elements composing it; the elements having atomic heat capacities lower than those required by the Dulong–Petit law retain these lower values in their compounds." #In studying organic compounds, Kopp found a regular relationship between boiling points and the number of CH2 groups present.See page 942 of Kopp–Neumann law The Kopp–Neumann law, named for Kopp and Franz Ernst Neumann, is a common approach for determining the specific heat ''C'' (in J·kg−1·K−1) of compounds using the following equation: C = \sum_^N C_i f_i, where ''N'' is the total number of compound constituents, and ''Ci'' and ''fi'' denote the specific heat and Mass fraction (chemistry), mass fraction of the ''i''-th constituent. This law works surprisingly well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hermann Kopp 01
Hermann or Herrmann may refer to: * Hermann (name), list of people with this name * Arminius, chieftain of the Germanic Cherusci tribe in the 1st century, known as Hermann in the German language * Éditions Hermann, French publisher * Hermann, Missouri, a town on the Missouri River in the United States ** Hermann AVA, Missouri wine region * The German SC1000 bomb of World War II was nicknamed the "Hermann" by the British, in reference to Hermann Göring * Herrmann Hall, the former Hotel Del Monte, at the Naval Postgraduate School, Monterey, California * Memorial Hermann Healthcare System, a large health system in Southeast Texas * The Herrmann Brain Dominance Instrument (HBDI), a system to measure and describe thinking preferences in people * Hermann station (other), stations of the name * Hermann (crater), a small lunar impact crater in the western Oceanus Procellarum * Hermann Huppen, a Belgian comic book artist * Hermann 19, an American sailboat design built by Ted He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hermann Franz Moritz Kopp
Hermann Franz Moritz Kopp (30 October 1817 – 20 February 1892), German chemist, was born at Hanau, where his father, Johann Heinrich Kopp (1777–1858), a physician, was professor of chemistry, physics and natural history at the local lyceum. After attending the gymnasium of his native town, he studied at Marburg and Heidelberg, and then, attracted by the fame of Liebig, went in 1839 to Gießen, where he became a ''privatdozent'' in 1841, and professor of chemistry twelve years later. In 1864 he was called to Heidelberg in the same capacity, and he remained there until his death. Kopp devoted himself especially to physico-chemical inquiries, and in the history of chemical theory his name is associated with several of the most important correlations of the physical properties of substances with their chemical constitution. Much of his work was concerned with specific volumes, the conception of which he set forth in a paper published when he was only twenty-two years of age; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dulong–Petit Law
The Dulong–Petit law, a thermodynamic law proposed by French physicists Pierre Louis Dulong and Alexis Thérèse Petit, states that the classical expression for the molar specific heat capacity of certain chemical elements is constant for temperatures far from the absolute zero. In modern terms, Dulong and Petit found that the heat capacity of a mole (unit), mole of many solid elements is about 3''R'', where ''R'' is the universal gas constant. The modern theory of the heat capacity of solids states that it is due to phonon, lattice vibrations in the solid. History Experimentally Pierre Louis Dulong and Alexis Thérèse Petit had found in 1819 that the heat capacity per weight (the mass-specific heat capacity) for 13 measured elements was close to a constant value, after it had been multiplied by a number representing the presumed relative atomic weight of the element. These atomic weights had shortly before been suggested by John Dalton and modified by Jacob Berzelius. Du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Franz Ernst Neumann
Franz Ernst Neumann (11 September 1798 – 23 May 1895) was a German mineralogist and physicist. He devised the first formulas to calculate inductance. He also formulated Neumann's law for molecular heat. In electromagnetism, he is credited for introducing the magnetic vector potential. Biography Early life Franz Ernst Neumann was born in Joachimsthal, Margraviate of Brandenburg (near Berlin), the son of Ernst Neumann, a farmer who became a state agent. His mother was a Countess who was not allowed to marry Ernst, and Neumann did not meet her mother until he was 10 years old. Neumann attended a Gymnasium in Berlin, where he excelled in mathematics. However his studies were interrupted by the war with France. In 1815 he paused his education at Berlin to serve as a volunteer in the Hundred Days against Napoleon, and was wounded in the Battle of Ligny. He later returned to finish his studies in Berlin. Subsequently, he enrolled at Berlin University in 1818 as a student of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat capacity or as the specific heat. More formally it is the heat capacity of a sample of the substance divided by the mass of the sample. The International System of Units, SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of of water by is , so the specific heat capacity of water is . Specific heat capacity often varies with temperature, and is different for each state of matter. Liquid water has one of the highest specific heat capacities among common substances, about at 20 °C; but that of ice, just below 0 °C, is only . The specific heat capacities of iron, granite, and hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Fraction (chemistry)
In chemistry, the mass fraction of a substance within a mixture is the ratio w_i (alternatively denoted Y_i) of the mass m_i of that substance to the total mass m_\text of the mixture. Expressed as a formula, the mass fraction is: : w_i = \frac . Because the individual masses of the ingredients of a mixture sum to m_\text, their mass fractions sum to unity: : \sum_^ w_i = 1. Mass fraction can also be expressed, with a denominator of 100, as percentage by mass (in commercial contexts often called ''percentage by weight'', abbreviated ''wt.%'' or ''% w/w''; see mass versus weight). It is one way of expressing the composition of a mixture in a dimensionless size; mole fraction (percentage by moles, mol%) and volume fraction ( percentage by volume, vol%) are others. When the prevalences of interest are those of individual chemical elements, rather than of compounds or other substances, the term ''mass fraction'' can also refer to the ratio of the mass of an element to the tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rule Of Mixtures
In materials science, a general rule of mixtures is a weighted mean used to predict various properties of a composite material . It provides a theoretical upper- and lower-bound on properties such as the elastic modulus, ultimate tensile strength, thermal conductivity, and electrical conductivity. In general there are two models, the ''rule of mixtures'' for axial loading (Voigt model), and the ''inverse rule of mixtures'' for transverse loading (Reuss model). For some material property E, the rule of mixtures states that the overall property in the direction parallel to the fibers could be as high as : E_\parallel = fE_f + \left(1-f\right)E_m The inverse rule of mixtures states that in the direction perpendicular to the fibers, the elastic modulus of a composite could be as low as :E_\perp = \left(\frac + \frac\right)^. where * f = \frac is the volume fraction of the fibers * E_\parallel is the material property of the composite parallel to the fibers * E_\perp is the materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick Seitz
Frederick Seitz (July 4, 1911 – March 2, 2008) was an American physicist, a pioneer of solid state physics, and climate change denier. Seitz was the 4th president of Rockefeller University from 1968 to 1978, and the 17th president of the National Academy of Sciences from 1962 to 1969. Seitz was the recipient of the National Medal of Science, NASA's Distinguished Public Service Award, and other honors. He founded the Frederick Seitz Materials Research Laboratory at the University of Illinois at Urbana–Champaign and several other material research laboratories across the United States. Seitz was also the founding chairman of the George C. Marshall Institute. Background and personal life Seitz was born in San Francisco on July 4, 1911. His mother was also from San Francisco and his father, after whom he was named, was born in Germany. Seitz graduated from Lick-Wilmerding High School in the middle of his senior year, and went on to study physics at Stanford University obtaining ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ASIN
Asin Thottumkal (born 26 October 1985), known mononyomusly as Asin, is an Indian former actress who appeared predominantly in Tamil cinema, Tamil, Hindi and Telugu language, Telugu films. Asin is a recipient of List of awards and nominations received by Asin, several accolades including a Filmfare Awards, Filmfare Award, two Filmfare Awards South and four South Indian International Movie Awards, SIIMA Awards. The Government of Tamil Nadu honoured her with the state's highest civilian award Kalaimamani, in 2009. Asin is considered as one of the leading South Indian actresses of the 2000s and is referred to as the "Queen of Kollywood". A trained Bharatanatyam dancer, Asin made her acting debut at 15 in Sathyan Anthikkad's Malayalam film ''Narendran Makan Jayakanthan Vaka'' (2001). Asin had her first commercial success with the Telugu language, Telugu film ''Amma Nanna O Tamila Ammayi'' in 2003, and won a Filmfare Award for Best Actress – Telugu, Filmfare Best Telugu Actress Aw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |