|
Johann Friedrich Wilhelm Adolf Von Baeyer
Johann Friedrich Wilhelm Adolf von Baeyer (; 31 October 1835 – 20 August 1917) was a German chemist who synthesised indigo and developed a nomenclature for cyclic compounds (that was subsequently extended and adopted as part of the IUPAC organic nomenclature). He was ennobled in the Kingdom of Bavaria in 1885 and was the 1905 recipient of the Nobel Prize in Chemistry.''Adolf von Baeyer: Winner of the Nobel Prize for Chemistry 1905 '' Armin de Meijere Angewandte Chemie International Edition Volume 44, Issue 48, Pages 7836 – 7840 2005''Abstract/ref> Family and education Baeyer was born in Berlin as the son of the noted geodesist and captain of the Royal Prussian Army Johann Jacob Baeyer and his wife Eugenie Baeyer née Hitzig (1807–1843). Both his parents were Lutherans at the time of his birth and he was raised in the Lutheran religion. His mother was the daughter of Julius Eduard Hitzig and a member of the originally Jewish Itzig family, and had converted to Christianity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bayer
Bayer AG (, commonly pronounced ; ) is a German multinational corporation, multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of business include pharmaceuticals; consumer healthcare products, agricultural chemicals, seeds and biotechnology products. The company is a component of the Euro Stoxx 50 stock market index. Bayer was founded in 1863 in Barmen as a partnership between dye salesman Friedrich Bayer and dyer Friedrich Weskott. As was common in this era, the company was established as a dyestuffs producer. The versatility of aniline chemistry led Bayer to expand their business into other areas, and in 1899 Bayer launched the compound acetylsalicylic acid under the trademarked name Aspirin. In 1904 Bayer received a trademark for the "Bayer Cross" logo, which was subsequently stamped onto each aspirin tablet, creating an iconic product that is still sold by Bayer. Ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Theodore Liebermann
Carl Theodore Liebermann (23 February 1842 – 28 December 1914) was a German chemist and student of Adolf von Baeyer. Life Liebermann first studied at the University of Heidelberg where Robert Wilhelm Bunsen was teaching. He then joined the group of Adolf von Baeyer at the University of Berlin where he received his PhD in 1865. Together with Carl Gräbe, Liebermann synthesised the orange-red dye alizarin in 1868. After his habilitation in 1870 he became professor at the University of Berlin after Adolf von Baeyer left for the University of Strasbourg. Shortly after Liebermann retired, in 1914, he died. Work In 1826, the French chemist Pierre Jean Robiquet had isolated from the root of a plant, madder, and defined the structure of, alizarin, a remarkable red dye. Liebermann's 1868 discovery that alizarin can be reduced to form anthracene, which is an abundant component in coal tar, opened the road for synthetic alizarin. The patent of Liebermann and Carl Gräbe for the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Otto Hahn
Otto Hahn (; 8 March 1879 – 28 July 1968) was a German chemist who was a pioneer in the fields of radioactivity and radiochemistry. He is referred to as the father of nuclear chemistry and father of nuclear fission. Hahn and Lise Meitner discovered radioactive isotopes of radium, thorium, protactinium and uranium. He also discovered the phenomena of atomic recoil and nuclear isomerism, and pioneered rubidium–strontium dating. In 1938, Hahn, Lise Meitner and Fritz Strassmann discovered nuclear fission, for which Hahn received the 1944 Nobel Prize for Chemistry. Nuclear fission was the basis for nuclear reactors and nuclear weapons. A graduate of the University of Marburg, Hahn studied under Sir William Ramsay at University College London and at McGill University in Montreal under Ernest Rutherford, where he discovered several new radioactive isotopes. He returned to Germany in 1906; Emil Fischer placed a former woodworking shop in the basement of the Chemical Institute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
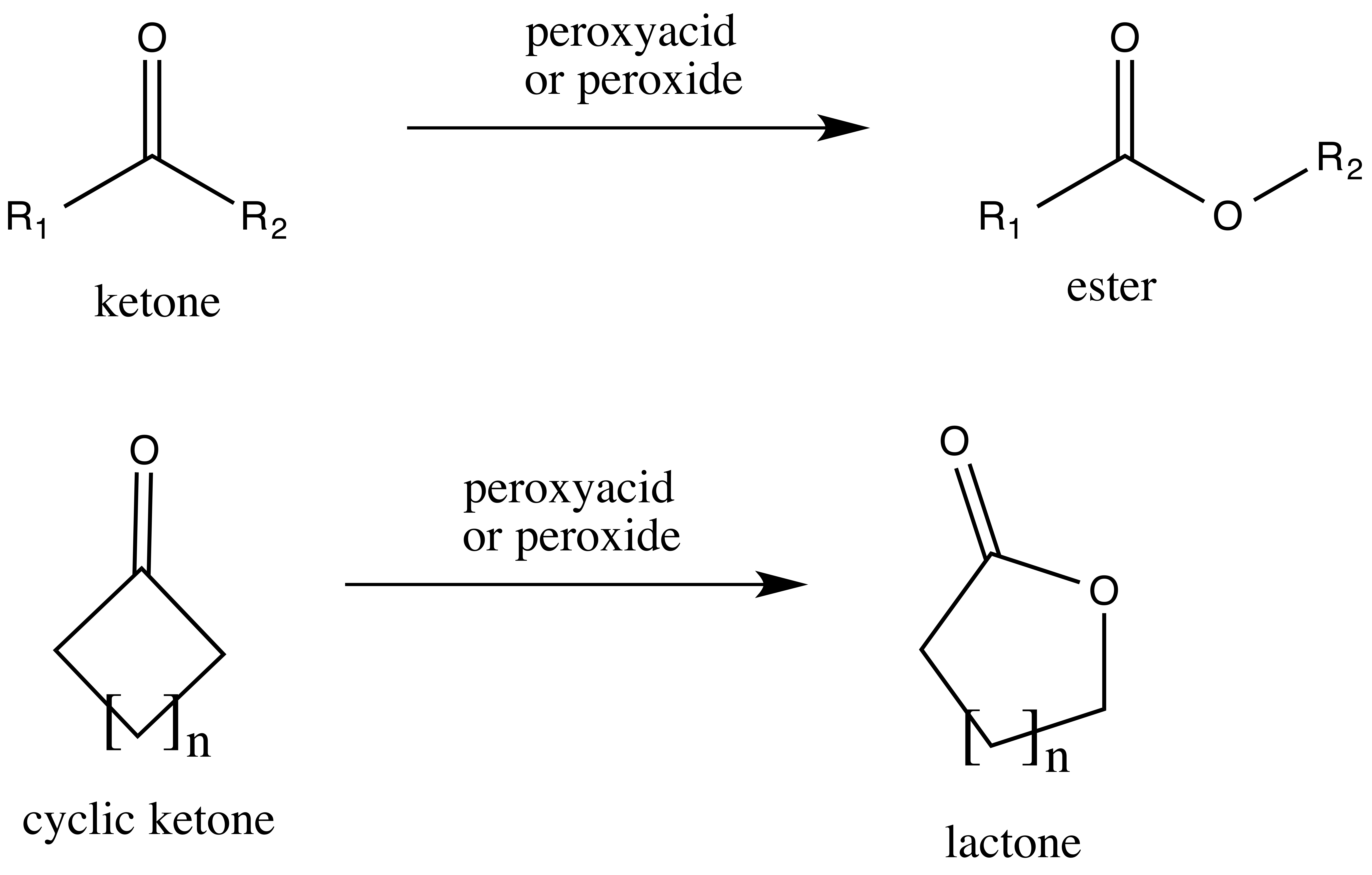
Baeyer–Villiger Oxidation
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899. Reaction mechanism In the first step of the reaction mechanism, the peroxyacid protonates the oxygen of the carbonyl group. This makes the carbonyl group more susceptible to be attacked by the peroxyacid. Next, the peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate. Through a concerted mechanism, one of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step. Finally, deprotonation of the oxocarbenium ion produces the ester. The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baeyer–Emmerling Indole Synthesis
The Baeyer–Emmerling indole synthesis is a method for synthesizing indole from a (substituted) ''ortho''-nitrocinnamic acid and iron powder in strongly basic solution. This reaction was discovered by Adolf von Baeyer and Adolph Emmerling in 1869. Reaction mechanism The reaction of iron powder with ''o''-nitrocinnamic acid reduces the nitro group to a nitroso. The nitrogen then condenses with a carbon on the alkene chain with loss of a molecule of water to form a ring. Decarboxylation gives indole. See also * Baeyer–Drewson indigo synthesis The Baeyer–Drewson indigo synthesis (1882) is an organic reaction in which indigo is prepared from 2-nitrobenzaldehyde and acetone The reaction was developed by von Baeyer in 1880 to produce the first synthetic indigo at laboratory ... References {{DEFAULTSORT:Baeyer-Emmerling Indole Synthesis Indole forming reactions Organic reactions Name reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baeyer–Drewson Indigo Synthesis
The Baeyer–Drewson indigo synthesis (1882) is an organic reaction in which indigo is prepared from 2-nitrobenzaldehyde and acetone The reaction was developed by von Baeyer in 1880 to produce the first synthetic indigo at laboratory scale. This procedure is not used at industrial scale. The reaction is classified as an aldol condensation. As a practical route to indigo, this method was displaced by routes from aniline Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aroma ....Elmar Steingruber "Indigo and Indigo Colorants" Ullmann's Encyclopedia of Industrial Chemistry 2004, Wiley-VCH, Weinheim. Mechanism Note In the English literature this reaction is usually called Baeyer–Drewson reaction, although the author of the original paper was called Drewsen. References E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Permanganate
Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and , an intensely pink to purple solution. Potassium permanganate is widely used in the chemical industry and laboratories as a strong oxidizing agent, and also as a medication for dermatitis, for cleaning wounds, and general disinfection. It is on the World Health Organization's List of Essential Medicines. In 2000, worldwide production was estimated at 30,000 tonnes. Properties Potassium permanganate is the potassium salt of the tetrahedral transition metal oxo complex permanganate, in which four O2- ligands are bound to a manganese(VII) center. Structure KMnO4 forms orthorhombic crystals with constants: ''a'' = 910.5 pm, ''b'' = 572.0 pm, ''c'' = 742.5 pm. The overall motif is similar to that for barium sulfate, with which it forms solid solutions. In the solid (as in solution), each MnO4− centre is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Von Baeyer Nomenclature
The von Baeyer nomenclature is a system for describing polycyclic hydrocarbons. The system was originally developed in 1900 by Adolf von Baeyer for bicyclic systemsAdolf Baeyer: ''Systematik und Nomenclatur bicyclischer Kohlenwasserstoffe.'' and in 1913 expanded by Eduard Buchner and Wilhelm Weigand for tricyclic systems. The system has been adopted and extended by the IUPAC as part of its nomenclature for organic chemistry. The modern version has been extended to cover more cases of compounds including an arbitrary number of cycles, heterocyclic compounds A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ... and unsaturated compounds. Extensive errata to this book has published online as: Extended Von Baeyer See also * Clar's Rule: Polycyclic aromatic hydrocarbon#Physicochemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photogeochemistry
Photogeochemistry merges photochemistry and geochemistry into the study of light-induced chemical reactions that occur or may occur among natural components of Earth's surface. The first comprehensive review on the subject was published in 2017 by the chemist and soil scientist Timothy A Doane, but the term photogeochemistry appeared a few years earlier as a keyword in studies that described the role of light-induced mineral transformations in shaping the biogeochemistry of Earth; this indeed describes the core of photogeochemical study, although other facets may be admitted into the definition. The domain of photogeochemistry The context of a photogeochemical reaction is implicitly the surface of Earth, since that is where sunlight is available (although other sources of light such as chemiluminescence would not be strictly excluded from photogeochemical study). Reactions may occur among components of land such as rocks, soil and detritus; components of surface water such as s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications. The color of its aqueous solutions is green by reflection and orange by transmission (its spectral properties are dependent on pH of the solution), as can be noticed in bubble levels, for example, in which fluorescein is added as a colorant to the alcohol filling the tube in order to increase the visibility of the air bubble contained within (thus enhancing the precision of the instrument). More concentrated solutions of fluorescein can even appear red (because under these conditions nearly all incident emission is re-absorbed by the solution). It is on the World Health Organization's List of Essential Medicines. Uses Fluorescein sodium, the sodium salt of fluorescein, is used extensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenolphthalein
Phenolphthalein ( ) is a chemical compound with the chemical formula, formula carbon, C20hydrogen, H14oxygen, O4 and is often written as "HIn", "HPh", "phph" or simply "Ph" in shorthand notation. Phenolphthalein is often used as an indicator in acid–base titrations. For this application, it turns colorless in acidic solutions and pink in base (chemistry), basic solutions. It belongs to the class of dyes known as phthalein dyes. Phenolphthalein is slightly soluble in water and usually is dissolved in Alcohol (chemistry), alcohols for use in experiments. It is a weak acid, which can lose H+ ions in solution. The nonionized phenolphthalein molecule is colorless and the double deprotonated phenolphthalein ion is Fuchsia (color), fuchsia. Further loss of proton in higher pH occurs slowly and leads to a colorless form. Phenolphthalein ion in concentrated sulfuric acid is orange red due to sulfonation. Uses pH indicator Phenolphthalein's common use is as an indicator in acid-base t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |