|
Jean-Claude Lorquet
Jean-Claude Lorquet (born 19 September 1935) is a professor of Theoretical Chemistry at the University of Liège. He is member of the International Academy of Quantum Molecular Science and author of over 100 scientific papers. Some of his students are also well known for their contribution to quantum chemistry and reactivity: Michèle Desouter-Lecomte, Bernard Ley, Françoise Remacle. Lorquet was born in Liège, Belgium. Important contributions * Theory of mass spectra. * Study of reaction paths and of dissociation mechanisms of electronically excited molecular ions. * Nonadiabatic interactions. * Avoided crossings and conical intersections. * Transition probabilities between two coupled potential energy surfaces. * Statistical calculation of rate constants of nonadiabatic reactions. * Validity of statistical theories of unimolecular reactions under collision-free conditions. * Study of intramolecular vibrational energy relaxation, of phase space sampling and of unimolecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theoretical Chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface of potential energy, molecular orbitals, orbital interactions, and molecule activation. Overview Theoretical chemistry unites principles and concepts common to all branches of chemistry. Within the framework of theoretical chemistry, there is a systematization of chemical laws, principles and rules, their refinement and detailing, the construction of a hierarchy. The central place in theoretical chemistry is occupied by the doctrine of the interconnection of the structure and properties of molecular systems. It uses mathematical and physical methods to explain the structures and dynamics of chemical systems and to correlate, understand, and predict their thermodynamic and kinetic properties. In the most general sense, it is explanation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
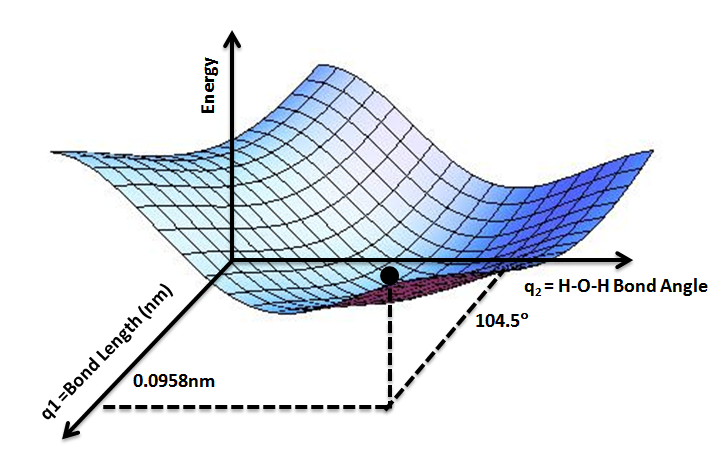
Potential Energy Surface
A potential energy surface (PES) describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a ''potential energy curve'' or energy profile. An example is the Morse/Long-range potential. It is helpful to use the analogy of a landscape: for a system with two degrees of freedom (e.g. two bond lengths), the value of the energy (analogy: the height of the land) is a function of two bond lengths (analogy: the coordinates of the position on the ground). The PES concept finds application in fields such as chemistry and physics, especially in the theoretical sub-branches of these subjects. It can be used to theoretically explore properties of structures composed of atoms, for example, finding the minimum energy shape of a molecule or computing the rates of a chemical reaction. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Belgian Chemists
Belgian may refer to: * Something of, or related to, Belgium * Belgians, people from Belgium or of Belgian descent * Languages of Belgium, languages spoken in Belgium, such as Dutch, French, and German *Ancient Belgian language, an extinct language formerly spoken in Gallia Belgica *Belgian Dutch or Flemish, a variant of Dutch *Belgian French, a variant of French *Belgian horse (other), various breeds of horse *Belgian waffle, in culinary contexts * SS ''Belgian'', a cargo ship in service with F Leyland & Co Ltd from 1919 to 1934 *''The Belgian'', a 1917 American silent film See also * *Belgica (other) Gallia Belgica was a province of the Roman Empire in present-day Belgium, Luxembourg, and the Netherlands. Belgica may also refer to: Places * Belgica Glacier, Antarctica * Belgica Guyot, an undersea tablemount off Antarctica * Belgica Mountain ... * Belgic (other) {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scientists From Liège
A scientist is a person who conducts scientific research to advance knowledge in an area of the natural sciences. In classical antiquity, there was no real ancient analog of a modern scientist. Instead, philosophers engaged in the philosophical study of nature called natural philosophy, a precursor of natural science. Though Thales (circa 624-545 BC) was arguably the first scientist for describing how cosmic events may be seen as natural, not necessarily caused by gods,Frank N. Magill''The Ancient World: Dictionary of World Biography'', Volume 1 Routledge, 2003 it was not until the 19th century that the term ''scientist'' came into regular use after it was coined by the theologian, philosopher, and historian of science William Whewell in 1833. In modern times, many scientists have advanced degrees in an area of science and pursue careers in various sectors of the economy such as academia, industry, government, and nonprofit environments.'''' History The roles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Living People
Related categories * :Year of birth missing (living people) / :Year of birth unknown * :Date of birth missing (living people) / :Date of birth unknown * :Place of birth missing (living people) / :Place of birth unknown * :Year of death missing / :Year of death unknown * :Date of death missing / :Date of death unknown * :Place of death missing / :Place of death unknown * :Missing middle or first names See also * :Dead people * :Template:L, which generates this category or death years, and birth year and sort keys. : {{DEFAULTSORT:Living people 21st-century people People by status ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1935 Births
Events January * January 7 – Italian premier Benito Mussolini and French Foreign Minister Pierre Laval conclude Franco-Italian Agreement of 1935, an agreement, in which each power agrees not to oppose the other's colonial claims. * January 12 – Amelia Earhart becomes the first person to successfully complete a solo flight from Hawaii to California, a distance of 2,408 miles. * January 13 – A plebiscite in the Saar (League of Nations), Territory of the Saar Basin shows that 90.3% of those voting wish to join Germany. * January 24 – The first canned beer is sold in Richmond, Virginia, United States, by Gottfried Krueger Brewing Company. February * February 6 – Parker Brothers begins selling the board game Monopoly (game), Monopoly in the United States. * February 13 – Richard Hauptmann is convicted and sentenced to death for the kidnapping and murder of Charles Lindbergh Jr. in the United States. * February 15 – The discovery and clinical development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonadiabatic Transition State Theory
Nonadiabatic transition state theory (NA-TST) is a powerful tool to predict rates of chemical reactions from a computational standpoint. NA-TST has been introduced in 1988 by Prof. J.C. Lorquet. In general, all of the assumptions taking place in traditional transition state theory In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. T ... (TST) are also used in NA-TST but with some corrections. First, a spin-forbidden reaction proceeds through the minimum energy crossing point (MECP) rather than through transition state (TS). Second, unlike TST, the probability of transition is not equal to unity during the reaction and treated as a function of internal energy associated with the reaction coordinate. At this stage non-relativistic couplings responsible for mixing between states is a driving ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autocorrelation Function
Autocorrelation, sometimes known as serial correlation in the discrete time case, is the correlation of a signal with a delayed copy of itself as a function of delay. Informally, it is the similarity between observations of a random variable as a function of the time lag between them. The analysis of autocorrelation is a mathematical tool for finding repeating patterns, such as the presence of a periodic signal obscured by noise, or identifying the missing fundamental frequency in a signal implied by its harmonic frequencies. It is often used in signal processing for analyzing functions or series of values, such as time domain signals. Different fields of study define autocorrelation differently, and not all of these definitions are equivalent. In some fields, the term is used interchangeably with autocovariance. Unit root processes, trend-stationary processes, autoregressive processes, and moving average processes are specific forms of processes with autocorrelation. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
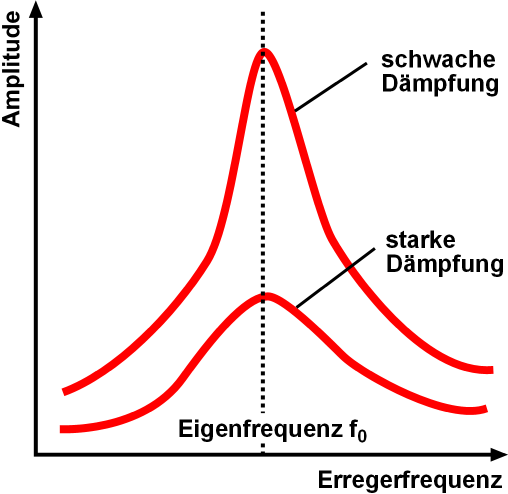
Resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies. Frequencies at which the response amplitude is a relative maximum are also known as resonant frequencies or resonance frequencies of the system. Small periodic forces that are near a resonant frequency of the system have the ability to produce large amplitude oscillations in the system due to the storage of vibrational energy. Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, orbital resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance (ESR) and reso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
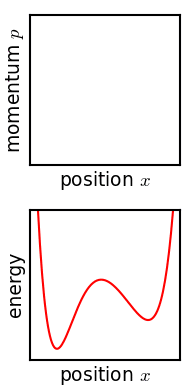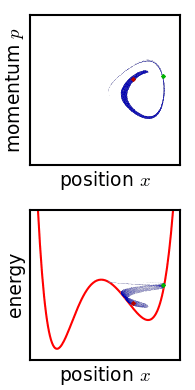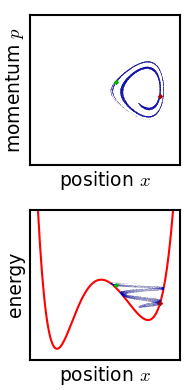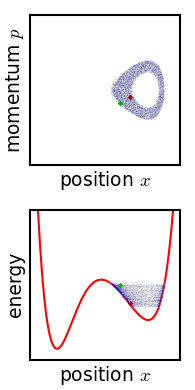
Phase Space
In dynamical system theory, a phase space is a space in which all possible states of a system are represented, with each possible state corresponding to one unique point in the phase space. For mechanical systems, the phase space usually consists of all possible values of position and momentum variables. It is the outer product of direct space and reciprocal space. The concept of phase space was developed in the late 19th century by Ludwig Boltzmann, Henri Poincaré, and Josiah Willard Gibbs. Introduction In a phase space, every degree of freedom or parameter of the system is represented as an axis of a multidimensional space; a one-dimensional system is called a phase line, while a two-dimensional system is called a phase plane. For every possible state of the system or allowed combination of values of the system's parameters, a point is included in the multidimensional space. The system's evolving state over time traces a path (a phase-space trajectory for the system) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rate Constant
In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction. For a reaction between reactants A and B to form product C the reaction rate is often found to have the form: r = k(T) mathrmm mathrm Here ''k''(''T'') is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reaction mechanism and can be determined experimentally. Elementary steps For an elementary step, there ''is'' a relationship between stoichiometry and rate law, as determined by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |