|
Janus Green B
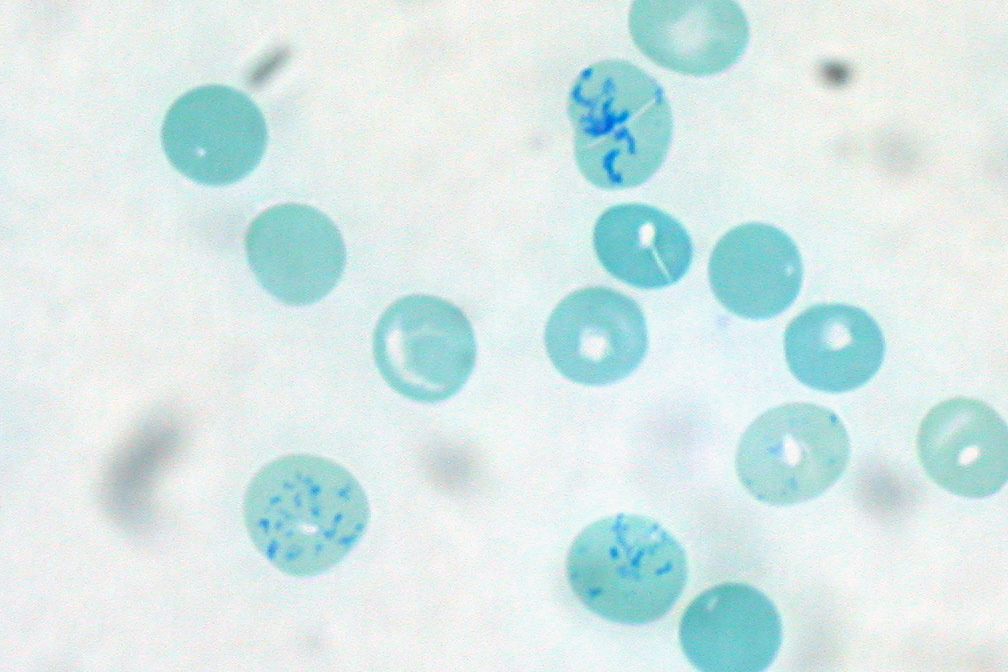
Janus Green B is a basic dye and vital stain used in histology. It is also used to stain mitochondria supravitally, as was introduced by Leonor Michaelis Leonor Michaelis (16 January 1875 – 8 October 1949) was a German biochemist, physical chemist, and physician, known for his work with Maud Menten on enzyme kinetics in 1913, as well as for work on enzyme inhibition, pH and quinones. Ear ... in 1900. The indicator Janus Green B changes colour according to the amount of oxygen present. When oxygen is present, the indicator oxidizes to a blue colour. In the absence of oxygen, the indicator is reduced and changes to a pink colour. References Staining dyes Azo dyes Phenazines Chlorides Aromatic amines Anilines Diethylamino compounds {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company that is owned by the German chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company is headquartered in St. Louis and has operations in approximately 40 countries. In 2015, the German chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they merged in August 1975. The company grew throughout the 1980s and 1990s, with significant expansion in fac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vital Stain
A vital stain in a casual usage may mean a stain that can be applied on living cells without killing them. Vital stains have been useful for diagnostic and surgical techniques in a variety of medical specialties. In supravital staining, living cells have been removed from an organism, whereas intravital staining is done by injecting or otherwise introducing the stain into the body. The term vital stain is used by some authors to refer to an intravital stain, and by others interchangeably with a supravital stain, the core concept being that the cell being examined is still alive. In a more strict sense, the term vital staining has a meaning contrasting with supravital staining. While in supravital staining the living cells take up the stain, in "vital staining" – the most accepted but apparently paradoxical meaning of this term, the living cells exclude the stain i.e. stain negatively and only the dead cells stain positively and thus viability can be assessed by counting the percen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these four principal tissue types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondrion
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, ''Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular organism, '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supravital Staining
Supravital staining is a method of staining used in microscopy to examine living cells that have been removed from an organism. It differs from intravital staining, which is done by injecting or otherwise introducing the stain into the body. Thus a supravital stain may have a greater toxicity, as only a few cells need to survive it a short while. The term "vital stain" is used by some authors to refer specifically to an intravital stain, and by others interchangeably with a supravital stain, the core concept being that the cell being examined is still alive. As the cells are alive and unfixed, outside the body, supravital stains are temporary in nature. The most common supravital stain is performed on reticulocytes using new methylene blue or brilliant cresyl blue, which makes it possible to see the reticulofilamentous pattern of ribosomes characteristically precipitated in these live immature red blood cells by the supravital stains. By counting the number of such cells the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leonor Michaelis
Leonor Michaelis (16 January 1875 – 8 October 1949) was a German biochemist, physical chemist, and physician, known for his work with Maud Menten on enzyme kinetics in 1913, as well as for work on enzyme inhibition, pH and quinones. Early life and education Leonor Michaelis was born in Berlin, Germany, on 16 January 1875, and graduated from the humanistic Koellnisches Gymnasium in 1893 after passing the Abiturienten Examen. He was Jewish. It was here that Michaelis's interest in physics and chemistry was first sparked as he was encouraged by his teachers to utilize the relatively unused laboratories at his school. With concerns about the financial stability of a pure scientist, he commenced his study of medicine at Berlin University in 1893. Among his instructors were Emil du Bois-Reymond for physiology, Emil Fischer for chemistry, and Oscar Hertwig for histology and embryology. During his time at Berlin University, Michaelis worked in the lab of Oscar Hertwig, even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staining Dyes
Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues (highlighting, for example, muscle fibers or connective tissue), cell populations (classifying different blood cells), or organelles within individual cells. In biochemistry, it involves adding a class-specific ( DNA, proteins, lipids, carbohydrates) dye to a substrate to qualify or quantify the presence of a specific compound. Staining and fluorescent tagging can serve similar purposes. Biological staining is also used to mark cells in flow cytometry, and to flag proteins or nucleic acids in gel electrophoresis. Light microscopes are used for viewing st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N-C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group, but some dyes called "disazo dyes" contain two azo groups, some dyes called "trisazo dyes" contain three azo groups and are or more. Azo dyes comprise 60-70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenazines
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in alcohol. Sulfuric acid dissolves it, forming a deep-red solution. Synthesis Classically phenazine are prepared by the reaction of nitrobenzene and aniline in the Wohl-Aue reaction. Other methods include: * pyrolysis of the barium salt of azobenzoate * oxidation of aniline with lead oxide * oxidation of dihydrophenazine, which is prepared by heating pyrocatechin with o-phenylenediamine. * oxidation of ortho-aminodiphenylamine with lead peroxide. Derivatives * The more complex phenazines, such as the naphthophenazines, naphthazines, and naphthotolazines, may be prepared by condensing ortho-diamines with ortho-quinones or by the oxidation of an ortho-diamine in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Amines
In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses anilines, but also many more complex aromatic rings and many amine substituents beyond . Such compounds occur widely. Aromatic amines are widely used as precursor to pesticides, pharmaceuticals, and dyes. Aromatic amines in textiles Since August 2012, the new standard EN 14362-1:2012 ''Textiles - Methods for determination of certain aromatic amines derived from azo colorants - Part 1: Detection of the use of certain azo colorants accessible with and without extracting the fibres'' is effective. It had been officially approved by the European Committee for Standardization (CEN) and supersedes the test standards EN 14362-1: 2003 and EN 14362-2: 2003. The standard describes a procedure to detect EU banned aromatic amines derived from azo colorants in textile fibres, including natural, man-made, regenerated, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |