|
JWH-098
JWH-098 is a synthetic cannabinoid receptor agonist from the naphthoylindole family. It is the indole 2-methyl derivative of a closely related compound JWH-081, but has markedly different affinity for the CB1 and CB2 receptors. While JWH-081 is around ten fold selective for CB1 over CB2, in JWH-098 this is reversed, and it is around four times weaker than JWH-081 at CB1 while being six times more potent at CB2, giving it a slight selectivity for CB2 overall. This makes JWH-098 a good example of how methylation of the indole 2-position in the naphthoylindole series tends to increase CB2 affinity, but often at the expense of CB1 binding. Legal status In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as JWH-098 are Schedule I Controlled Substances. JWH-098 is illegal in Russia, Sweden, and the UK, [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH Cannabinoids
The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids. Some of those are: [Baidu] |
JWH-081
JWH-081 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. With a Ki of 1.2nM it is fairly selective for the CB1 subtype, its affinity at this subtype is measured at approximately 10x the affinity at CB2(12.4nM). It was discovered by and named after John W. Huffman. JWH-081 may be neurotoxic to animals when administered in high doses. Legal status In the United States, JWH-081 is a Schedule I Controlled Substance. As of October 2015, JWH-081 is a controlled substance in China. See also *JWH-018 *JWH-098 *JWH-164 *JWH-198 *JWH-210 JWH-210 is an analgesic chemical from the naphthoylindole family, which acts as a potent cannabinoid agonist at both the CB1 and CB2 receptors, with Ki values of 0.46 nM at CB1 and 0.69 nM at CB2. It is one of the most potent 4-substi ... References Designer drugs JWH cannabinoids Naphthoylindoles Phenol ethers CB1 receptor agonists {{canna ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
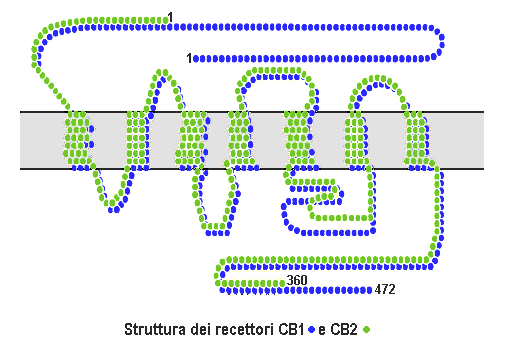
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Naphthoylindole
Naphthoylindoles are a class of synthetic cannabinoids. See also * Structural scheduling of synthetic cannabinoids To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general (or Markush) structure as opposed to their specific identity. In this way new analogs are already cont ... References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 1
Cannabinoid receptor type 1 (CB1), also known as cannabinoid receptor 1, is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by: endocannabinoids, a group of retrograde neurotransmitters that include anandamide and 2-arachidonoylglycerol (2-AG); plant phytocannabinoids, such as the compound THC which is an active ingredient of the psychoactive drug cannabis; and, synthetic analogs of THC. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin (THCV). The primary endogenous agonist of the human CB1 receptor is anandamide. Structure The CB1 receptor shares the structure characteristic of all G-protein-coupled receptors, possessing seven transmembrane domains connected by three extracellular and three intracellular loops, an extracellular N-terminal tail, and an intracellular C-terminal tail. The receptor may exist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor 2
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Schedule I Controlled Substance
This is the list of Schedule I drugs as defined by the United States Controlled Substances Act. 21 CFRbr>1308.11(CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for drugs to be placed in this schedule: # The drug or other substance has a high potential for abuse. # The drug or other substance has no currently accepted medical use in treatment in the United States. # There is a lack of accepted safety for use of the drug or other substance under medical supervision. Except as specifically authorized, it is illegal for any person: # to manufacture, distribute, or dispense, or possess with intent to manufacture, distribute, or dispense, a controlled substance; or # to create, distribute, dispense, or possess with intent to distribute or dispense, a counterfeit substance. Additional substances are added to the list by the Secretary of Health and Human Services pursuant to 21 CFR 1308.49. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-007
JWH-007 is an analgesic chemical from the naphthoylindole family, which acts as a cannabinoid agonist at both the CB1 and CB2 receptors. It was first reported in 1994 by a group including the noted cannabinoid chemist John W. Huffman. It was the most active of the first group of ''N''-alkyl naphoylindoles discovered by the team led by John W Huffman, several years after the family was initially described with the discovery of the ''N''-morpholinylethyl compounds pravadoline (WIN 48,098), JWH-200 (WIN 55,225) and WIN 55,212-2 by the Sterling Winthrop group. Several other ''N''-alkyl substituents were found to be active by Huffman's team including the ''n''-butyl, ''n''-hexyl, 2-heptyl, and cyclohexylethyl groups, but it was subsequently determined that the 2-methyl group on the indole ring is not required for CB1 binding, and tends to increase affinity for CB2 instead. Consequently, the 2-desmethyl derivative of JWH-007, JWH-018, has slightly higher binding affinity for CB1, wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthoylindoles
Naphthoylindoles are a class of synthetic cannabinoids. See also * Structural scheduling of synthetic cannabinoids To combat the illicit synthetic cannabinoid industry many jurisdictions have created a system to control these cannabinoids through their general (or Markush) structure as opposed to their specific identity. In this way new analogs are already cont ... References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Ethers
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |