|
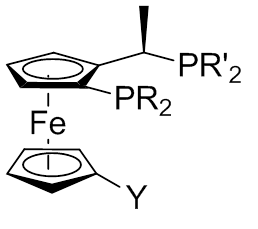
Josiphos Ligands
A Josiphos ligand is a type of chiral diphosphine which has been modified to be substrate (chemistry), substrate-specific; they are widely used for enantioselective synthesis.[3]H-U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer and A. Togni, Top. Catal ., 2002, 19, 3. They are named after the technician who made the first one, Josi Puleo. Applications Homogeneous catalysis is often used for Enantioselective synthesis, enantioselective transformations. The ligands carry chiral information and thus they are modified for individual substrates. Ligands can also influence the chemoselectivity of the catalyst. The Josiphos ligands, often called privileged ligands, are important because of their ability to give high yields in enantioselective synthesis. Josiphos ligands were developed in the 1990s by Antonio Togni in studies on Ferrocene, ferrocenyl ligands previously discovered by T. Hayashi (1986). These studies focused of an Au(I)-catalyzed aldol reaction at The Central Rese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymmetric Hydrogenation
Asymmetric hydrogenation is a chemical reaction that adds two atoms of Hydrogen atom, hydrogen to a target (substrate) molecule with three-dimensional Enantioselective synthesis, spatial selectivity. Critically, this selectivity does not come from the target molecule itself, but from other reagents or catalysts present in the reaction. This allows spatial information (what chemists refer to as chirality) to transfer from one molecule to the target, forming the product as a single enantiomer. The chiral information is most commonly contained in a catalyst and, in this case, the information in a single molecule of catalyst may be transferred to many substrate molecules, amplifying the amount of chiral information present. Similar processes occur in nature, where a chiral molecule like an enzyme can catalyse the introduction of a chiral centre to give a product as a single enantiomer, such as amino acids, that a cell needs to function. By imitating this process, chemists can generate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroformylation Of Styrene
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: Production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and drugs. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry. The process entails treatment of an alkene typically with high pressures (between 10 and 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. In one variation, formaldehyd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HB Of Styrene
HB or Hb may refer to: Academia * H-b index, an extension of the h-index used in determining academic impact * H-B Woodlawn, a secondary education program in Arlington, Virginia, US * Hathaway Brown School, an all-girls private school in Shaker Heights, Ohio, US Arts and media * HB (band), a Finnish Christian symphonic metal musical group * Hanna-Barbera, a cartoon studio, later folded into Warner Bros. Animation and Cartoon Network Studios * Heaven Below, an American rock band *Helluva Boss, an adult animated TV show Businesses and brands * HB (car), a 1920s automobile * HB (cigarette), a German brand of cigarettes * HB Construction, a private US general contractor construction business * HB Ice Cream, an Irish brand * Asia Atlantic Airlines (IATA code HB) * Hamilton Bradshaw, a London-based private equity firm * Hampton and Branchville Railroad (H&B) * Holland & Barrett (H&B), a UK health food shop chain * Hasbro, an American toy company Places * Bremen (state) (license plate: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ugi's Amine
Ugi’s amine is a chemical compound named for the chemist who first reported its synthesis in 1970, Ivar Karl Ugi, Ivar Ugi. It is a ferrocene derivative (chemistry), derivative. Since its first report, Ugi’s amine has found extensive use as the synthetic precursor to a large number of metal Ligand, ligands that bear planar chirality. These ligands have since found extensive use in a variety of catalytic reactions. The compound may exist in either the 1''S'' or 1''R'' isomer, both of which have synthetic utility and are commercially available. Most notably, it is the synthetic precursor to the Josiphos ligands, Josiphos class of ligands. History In 1967, Schlӧg repurposed the term “planar chirality” for use in substituted ferrocene terminology, which is necessary for ferrocenes in which ferrocene's innate plane of symmetry is broken by introducing two substituents to one of its ring. Nozaki, ''et al.'' demonstrated that a ferrocene derivative bearing a chiral amine subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Negishi Coupling
The Negishi coupling is a widely employed transition metal catalyzed cross-coupling reaction. The reaction couples organic halides or triflates with organozinc compounds, forming carbon-carbon bonds (C-C) in the process. A palladium (0) species is generally utilized as the metal catalyst, though nickel is sometimes used. A variety of nickel catalysts in either Ni0 or NiII oxidation state can be employed in Negishi cross couplings such as Ni(PPh3)4, Ni(acac)2, Ni(COD)2 etc. : :* The leaving group X is usually chloride, bromide, or iodide, but triflate and acetyloxy groups are feasible as well. X = Cl usually leads to slow reactions. :* The organic residue R = alkenyl, aryl, allyl, alkynyl or propargyl. :* The halide X' in the organozinc compound can be chloride, bromine or iodine and the organic residue R' is alkenyl, aryl, allyl, alkyl, benzyl, homoallyl, and homopropargyl. :* The metal M in the catalyst is nickel or palladium :* The ligand L in the catalyst can be triphenylp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Chloride
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications. Preparation The two main preparatory routes to aryl halides are direct halogenation and via diazonium salts. Direct halogenation In the Friedel-Crafts halogenation, Lewis acids serve as catalysts. Many metal chlorides are used, examples include iron(III) chloride or aluminium chloride. The most important aryl halide, chlorobenzene is produced by this route. Monochlorination of benzene is always accompanied by formation of the dichlorobenzene derivatives. Arenes with electron donating groups react with halogens even in the absence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |