|
Isotoluene
The isotoluenes in organic chemistry are the non-aromatic toluene isomers with an exocyclic double bond. They are of some academic interest in relation to aromaticity and isomerisation mechanisms. The three basic isotoluenes are ''ortho''-isotoluene or ''5-methylene-1,3-cyclohexadiene'' (here labelled 1); ''para''-isotoluene (2); and ''meta''-isotoluene (3). Another structural isomer is the bicyclic compound ''5-methylenebicyclo .2.0hexene'' (4). The ''o''- and ''p''-isotoluenes isomerise to toluene, a reaction driven by aromatic stabilisation. It is estimated that these compounds are 96 kJ mol−1 less stable. The isomerisation of ''p''-isotoluene to toluene takes place at 100 °C in benzene with bimolecular reaction kinetics by an intermolecular free radical reaction. The intramolecular isomerisation, a 1,3-sigmatropic reaction, is unfavorable because an antarafacial mode is enforced. Other dimer radical reaction products are formed as well. The ''ortho''-isomer is found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentacene
Pentacene () is a polycyclic aromatic hydrocarbon consisting of five linearly-fused benzene () rings. This highly conjugated compound is an organic semiconductor. The compound generates excitons upon absorption of ultra-violet ( UV) or visible light; this makes it very sensitive to oxidation. For this reason, this compound, which is a purple powder, slowly degrades upon exposure to air and light. Structurally, pentacene is one of the linear acenes, the previous one being tetracene (four fused benzene rings) and the next one being hexacene (six fused benzene rings). In August 2009, a group of researchers from IBM published experimental results of imaging a single molecule of pentacene using an atomic force microscope. In July 2011, they used a modification of scanning tunneling microscopy to experimentally determine the shapes of the highest occupied and lowest unoccupied molecular orbitals. In 2012, pentacene-doped ''p''-terphenyl was shown to be effective as the amplifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
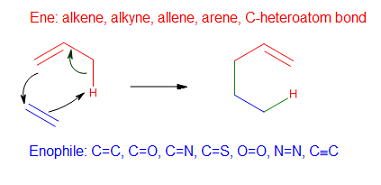
Ene Reaction
In organic chemistry, the ene reaction (also known as the Alder-ene reaction by its discoverer Kurt Alder in 1943) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position. This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Many useful Lewis acid-catalyzed ene reactions have been also developed, which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products. Ene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is to be contrasted with chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History In 1864, Peter Waage and Cato Guldberg pioneered the development of chemical kinetics by formulating the law of mass action, which states that the speed of a chemical reaction is proportional to the quantity of the reacting substances.C.M. Guldberg and P. Waage,"Studies Concerning Affinity" ''Forhandlinger i Videnskabs-Selskabet i Christiania'' (1864), 35P. W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to oxygen and water vapour and has a relatively low melting point. Polystyrene is one of the most widely used plastics, the scale of its production being several million tonnes per year. Polystyrene can be naturally transparent, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and in the jewel cases used for storage of optical discs such as CDs and occasionally DVDs), containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records. As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple free-radical reaction, radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. Alkanes can also be polymerized, but only with the help of strong acids. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides. Main types of initiation reaction *Halogens undergo homolytic fission relatively easily. Chlorine, for example, gives two chlorine radicals (Cl•) by irradiation with ultraviolet light. This process is used for chlorination of alkanes. *Azo compounds (R- N=N-R') can be the precursor of two carbon-centered radicals (R• and R'•) and nitrogen gas upon heating and/or by irradiation. For example, AIBN and ABCN yield isobutyronitrile and cyclohexanecarbonitrile radicals, respectively. : *Organic peroxides each have a peroxide bond (- O-O-), which is readi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concerted Reaction
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or other unstable high energy intermediates are not involved. Concerted reaction rates tend not to depend on solvent polarity ruling out large buildup of charge in the transition state. The reaction is said to progress through a concerted mechanism as all bonds are formed and broken ''in concert''. Pericyclic reactions, the S2 reaction, and some rearrangements - such as the Claisen rearrangement - are concerted reactions. The rate of the SN2 reaction is second order overall due to the reaction being bimolecular (i.e. there are two molecular species involved in the rate-determining step). The reaction does not have any intermediate steps, only a transition state In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antarafacial
Antarafacial ( Woodward-Hoffmann symbol a) and suprafacial (s) are two topological concepts in organic chemistry describing the relationship between two simultaneous chemical bond making and/or bond breaking processes in or around a reaction center. The reaction center can be a p- or sp''n-''orbital (Woodward-Hoffmann symbol ω), a conjugated system (π) or even a sigma bond (σ). * The relationship is ''antarafacial'' when opposite faces of the π system or isolated orbital are involved in the process (think ''anti''). For a σ bond, it corresponds to involvement of one "interior" lobe and one "exterior" lobe of the bond. * The relationship is ''suprafacial'' when the same face of the π system or isolated orbital are involved in the process (think ''syn''). For a σ bond, it corresponds to involvement of two "interior" lobes or two "exterior" lobes of the bond. The components of all pericyclic reactions, including sigmatropic reactions and cycloadditions, and electrocyclizatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_STM.jpg)