|
Isopentane
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula or . Isopentane is an extremely volatile and extremely flammable liquid at room temperature and pressure. It is also the least dense liquid at standard conditions. The normal boiling point is just a few degrees above room temperature and isopentane will readily boil and evaporate away on a warm day. Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane, but it is a significant component of natural gasoline.Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States', Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages. Nomenclature The traditional name isopentane was still retained in the 1993 IUPAC recommendations, but is no longer recommended according ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry, an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like pentacontane () or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines alkanes as "acyclic branched or unbranched hydrocarbons having the general formula , and therefore consisting entirely of hydrogen atoms and saturated carbon atoms". However, some sources use the term to denote ''any'' saturated hydrocarbon, including those that are either monocyclic (i.e. the cycloalkanes) or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octane Rating
An octane rating, or octane number, is a standard measure of a fuel's ability to withstand compression in an internal combustion engine without detonating. The higher the octane number, the more compression the fuel can withstand before detonating. Octane rating does not relate directly to the power output or the energy content of the fuel per unit mass or volume, but simply indicates gasoline's capability against compression. Whether or not a higher octane fuel improves or impairs an engine's performance depends on the design of the engine. In broad terms, fuels with a higher octane rating are used in higher-compression gasoline engines, which may yield higher power for these engines. Such higher power comes from the fuel's higher compression by the engine design, and not directly from the gasoline. In contrast, fuels with lower octane (but higher cetane numbers) are ideal for diesel engines because diesel engines (also called compression-ignition engines) do not compress the fue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutane
Isobutane, also known as ''i''-butane, 2-methylpropane or methylpropane, is a chemical compound with molecular formula HC(CH3)3. It is an isomer of butane. Isobutane is a colourless, odourless gas. It is the simplest alkane with a tertiary carbon atom. Isobutane is used as a precursor molecule in the petrochemical industry, for example in the synthesis of isooctane. Production Isobutane is obtained by isomerization of butane. : Uses Isobutane is the principal feedstock in alkylation units of refineries. Using isobutane, gasoline-grade "blendstocks" are generated with high branching for good combustion characteristics. Typical products created with isobutane are 2,4-dimethylpentane and especially 2,2,4-trimethylpentane. Solvent In the Chevron Phillips slurry process for making high-density polyethylene, isobutane is used as a diluent. As the slurried polyethylene is removed, isobutane is "flashed" off, and condensed, and recycled back into the loop reactor for this purpose. Prec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Normal Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. A liquid at low pressure has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Gasoline
Natural gasoline is a liquid hydrocarbon mixture condensed from natural gas, similar to common gasoline (petrol) derived from petroleum. The chemical composition of natural gasoline is mostly five- and six-carbon alkanes ( pentanes and hexanes) with smaller amounts of alkanes with longer chains.Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". ''International Journal of Thermophysics'', volume 35, pages 1450–1464. . Quote: "ethane 2.4% w/w, butane 1.3%, pentane 67.1%, hexane 22.0%, heptane 5.7%, and octane 1.5 %". It contains significant amounts of isopentane (methyl butane) , which is rare in the petroleum product.Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States', Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages. Its boiling point is within the standard range for gasoline, and its vapor press ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Isomerism
In chemistry, a structural isomer (or constitutional isomer in the IUPAC nomenclature) of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept. For example, butanol , methyl propyl ether , and diethyl ether have the same molecular formula but are three distinct structural isomers. The concept applies also to polyatomic ions with the same total charge. A classical example is the cyanate ion and the fulminate ion . It is also extended to ionic compounds, so that (for example) ammonium cyanate and urea are considered structural isomers,William F. Bynum, E. Janet Browne, Roy Porter (2014): ''Dictionary of the History of Science''. 530 pages. and so are methylammonium formate and ammonium acetate . Structural isomerism is the most radical type of isomerism. It is opposed to stereoisomerism, in which the atoms and bonding scheme ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Ethyl-1-butanol
2-Ethyl-1-butanol (IUPAC name: 2-ethylbutan-1-ol) is an organic chemical compound. It can be used to facilitate the separation of ethanol from water, which form an azeotrope that otherwise limits the maximum ethanol concentration. Reactions 2-Ethyl-1-butanol is manufactured industrially by the aldol condensation of acetaldehyde and butyraldehyde, followed by hydrogenation. It may also be prepared by the Guerbet reaction. Properties and applications The branching in 2-ethyl-1-butanol makes it harder to crystalize due to packing disruption, which results in a very low freezing point. Esters of 2-ethyl-1-butanol are similarly effected and it therefore finds application as a feedstock in the production of plasticizers and lubricants, where its presence helps reduce viscosity and lower freezing points. See also *2-Ethylhexanol 2-Ethylhexanol (abbreviated 2-EH) is an organic compound with formula CHO. It is a branched, eight-carbon chiral alcohol (chemistry), alcohol. It is a colorle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Ethylpentane
3-Ethylpentane (C7H16) is a branched saturated hydrocarbon. It is an alkane, and one of the many structural isomers of heptane, consisting of a five carbon chain with a two carbon branch at the middle carbon. An example of an alcohol Alcohol most commonly refers to: * Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom * Alcohol (drug), an intoxicant found in alcoholic drinks Alcohol may also refer to: Chemicals * Ethanol, one of sev ... derived from 3-ethylpentane is the tertiary alcohol 3-ethylpentan-3-ol.Ritchie, R and Gent, D: ''OCR Chemistry AS'', page 151. Heinemann, 2007. References Alkanes {{Hydrocarbon-stub it has 5 different skeletal structural isomers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
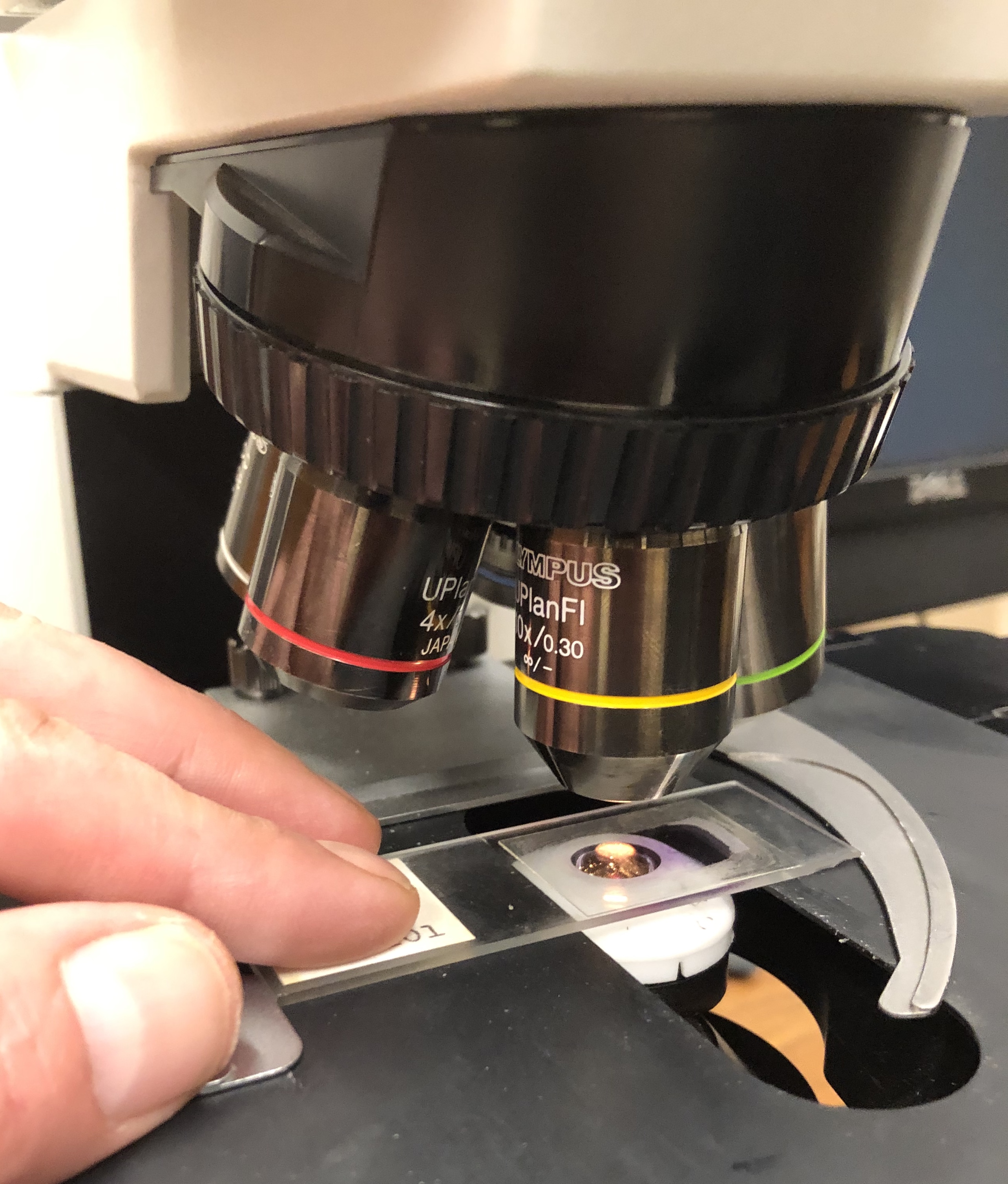
Histology
Histology, also known as microscopic anatomy or microanatomy, is the branch of biology which studies the microscopic anatomy of biological tissues. Histology is the microscopic counterpart to gross anatomy, which looks at larger structures visible without a microscope. Although one may divide microscopic anatomy into ''organology'', the study of organs, ''histology'', the study of tissues, and ''cytology'', the study of cells, modern usage places all of these topics under the field of histology. In medicine, histopathology is the branch of histology that includes the microscopic identification and study of diseased tissue. In the field of paleontology, the term paleohistology refers to the histology of fossil organisms. Biological tissues Animal tissue classification There are four basic types of animal tissues: muscle tissue, nervous tissue, connective tissue, and epithelial tissue. All animal tissues are considered to be subtypes of these four principal tissue types ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tissue (biology)
In biology, tissue is a biological organizational level between cells and a complete organ. A tissue is an ensemble of similar cells and their extracellular matrix from the same origin that together carry out a specific function. Organs are then formed by the functional grouping together of multiple tissues. The English word "tissue" derives from the French word "tissu", the past participle of the verb tisser, "to weave". The study of tissues is known as histology or, in connection with disease, as histopathology. Xavier Bichat is considered as the "Father of Histology". Plant histology is studied in both plant anatomy and physiology. The classical tools for studying tissues are the paraffin block in which tissue is embedded and then sectioned, the histological stain, and the optical microscope. Developments in electron microscopy, immunofluorescence, and the use of frozen tissue-sections have enhanced the detail that can be observed in tissues. With these tools, the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Ice
Dry ice is the solid form of carbon dioxide. It is commonly used for temporary refrigeration as CO2 does not have a liquid state at normal atmospheric pressure and sublimates directly from the solid state to the gas state. It is used primarily as a cooling agent, but is also used in fog machines at theatres for dramatic effects. Its advantages include lower temperature than that of water ice and not leaving any residue (other than incidental frost from moisture in the atmosphere). It is useful for preserving frozen foods (such as ice cream) where mechanical cooling is unavailable. Dry ice sublimates at at Earth atmospheric pressure. This extreme cold makes the solid dangerous to handle without protection from frostbite injury. While generally not very toxic, the outgassing from it can cause hypercapnia (abnormally elevated carbon dioxide levels in the blood) due to buildup in confined locations. Properties Dry ice is the solid form of carbon dioxide (CO2), a molecule co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neopentane
Neopentane, also called 2,2-dimethylpropane, is a double-branched-chain alkane with five carbon atoms. Neopentane is a flammable gas at room temperature and pressure which can condense into a highly volatile liquid on a cold day, in an ice bath, or when compressed to a higher pressure. Neopentane is the simplest alkane with a quaternary carbon, and has achiral tetrahedral symmetry. It is one of the three structural isomers with the molecular formula C5H12 (pentanes), the other two being ''n''-pentane and isopentane. Out of these three, it is the only one to be a gas at standard conditions; the others are liquids. Nomenclature The traditional name neopentane was still retained in the 1993 IUPAC recommendations, but is no longer recommended according to the 2013 recommendations. The preferred IUPAC name is the systematic name 2,2-dimethylpropane, but the substituent numbers are superfluous because it is the only possible “dimethylpropane”. A neopentyl substituent, often ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |