|
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful representations. They are sus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosylmethyl Isocyanide
TosMIC (toluenesulfonylmethyl isocyanide) is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide groups. It is a colourless solid that, unlike many isocyanides, is odorless. It is prepared by dehydration of the related formamide derivative. It is used in the Van Leusen reaction which is used to convert aldehydes to nitriles or in the preparation of oxazoles and imidazole Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...s. The versatility of TosMIC in organic synthesis has been documented. It is a fairly strong carbon acid, with an estimated p''K''a of 14 (compared to 29 for methyl tolyl sulfone), the isocyano group acting as an electron acceptor of strength comparable to an ester group. Further reading * References {{reflist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Isocyanide
Methyl isocyanide or isocyanomethane is an organic compound and a member of the isocyanide family. This colorless liquid is isomeric to methyl cyanide (acetonitrile), but its reactivity is very different. In contrast to the faintly sweet, ethereal odor of acetonitrile, the smell of methyl isocyanide, like that of other simple volatile isocyanides, is distinctly penetrating and vile. Methyl isocyanide is mainly used for making 5-membered heterocyclic rings. The C-N distance in methyl isocyanide is very short, 1.158 Å as is characteristic of isocyanides. Preparation and uses Methyl isocyanide was first prepared by A. Gautier by reaction of silver cyanide with methyl iodide Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one h .... The common method for preparing methyl isocyanides is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Oxychloride
Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula . It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride. It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate. Structure Like phosphate, is tetrahedral in shape. It features three P−Cl bonds and one strong P=O double bond, with an estimated bond dissociation energy of 533.5 kJ/mol. On the basis of bond length and electronegativity, the Schomaker-Stevenson rule suggests that the double bond form is dominant, in contrast with the case of . The P=O bond involves the donation of the lone pair electrons on oxygen ''p''-orbitals to the antibonding combinations associated with phosphorus-chlorine bonds, thus constituting ''π'' bonding. Phosphoryl chloride exists as neutral molecules in the solid, liquid and gas s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
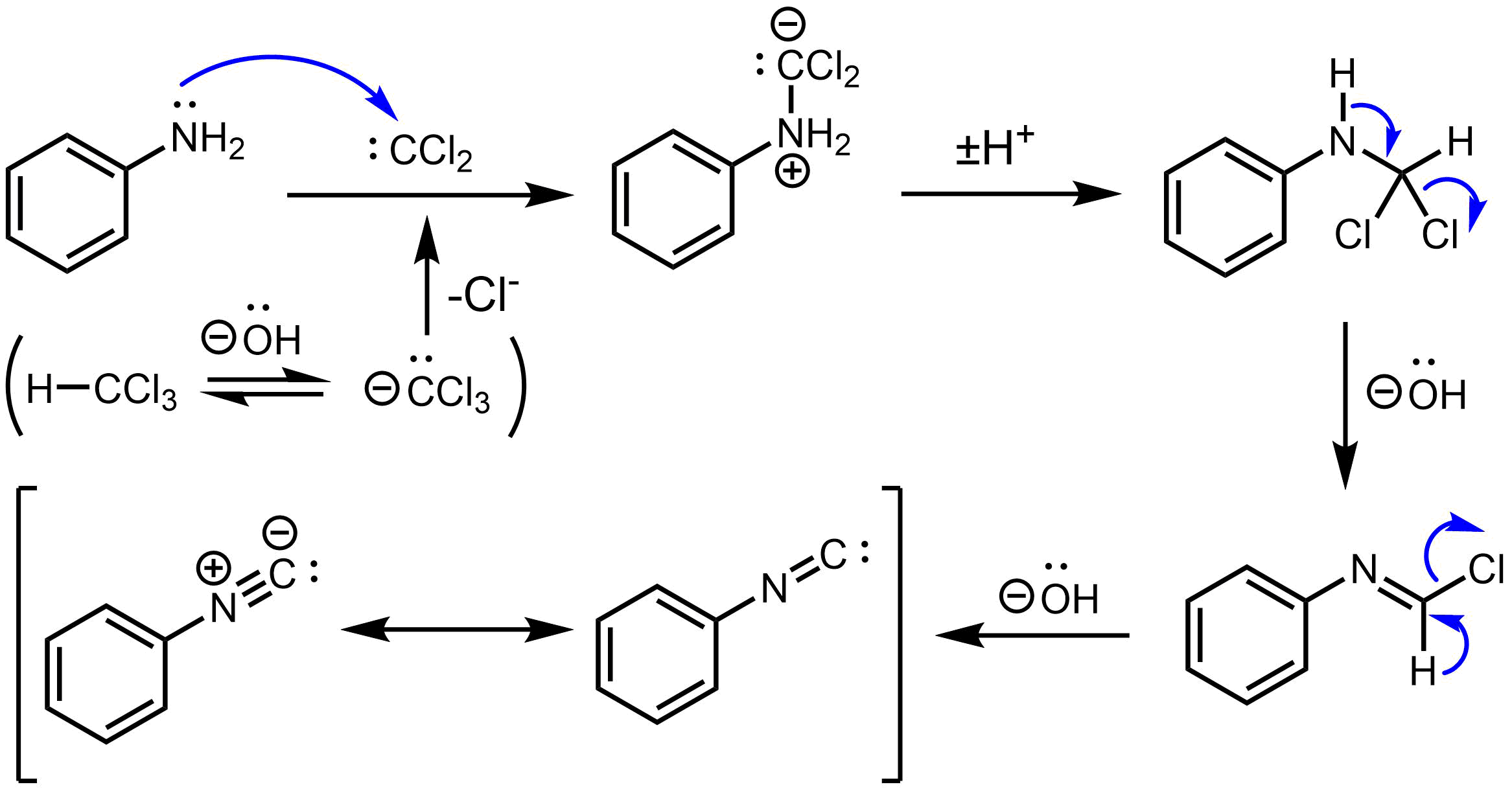
Carbylamine Reaction
The carbylamine reaction (also known as the Hoffmann isocyanide synthesis) is the synthesis of an isocyanide by the reaction of a primary amine, chloroform, and base. The conversion involves the intermediacy of dichlorocarbene. Illustrative is the synthesis of ''tert''-butyl isocyanide from ''tert''-butylamine in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride. :Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3 H2O Similar reactions have been reported for aniline. It is used to prepare secondary amines. Test for primary amines As it is only effective for primary amines, the carbylamine reaction can be used as a chemical test for their presence. In this context, the reaction is also known as Saytzeff's isocyanide test. In this reaction, the analyte is heated with alcoholic potassium hydroxide and chloroform. If a primary amine is present, the isocyanide (carbylamine) is formed, as indicated by a foul odour. The carbylamine test does ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroform
Chloroform, or trichloromethane, is an organic compound with chemical formula, formula Carbon, CHydrogen, HChlorine, Cl3 and a common organic solvent. It is a colorless, strong-smelling, dense liquid produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic, and sedative when inhaled or ingested. Structure The molecule adopts a tetrahedral molecular geometry with C3v symmetry group, symmetry. Natural occurrence The total global flux of chloroform through the environment is approximately tonnes per year, and about 90% of emissions are natural in origin. Many kinds of seaweed produce chloroform, and fungi are believed to produce chloroform in soil. Abiotic processes are also believed to contribute to natural chloroform productions in soils although the mechanism is still unclear. Chloroform volatilizes readily from soil and surface water and undergoes degradation in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest molecule of the oxocarbon family. In coordination complexes the carbon monoxide ligand is called carbonyl. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds, when insufficient oxygen or heat is present to produce carbon dioxide. There are also numerous environmental and biological sources that generate and emit a significant amount of carbon monoxide. It is important in the production of many compounds, including drugs, fragrances, and fuels. Upon emission into the atmosphere, carbon monoxide affects several processes that contribute to climate change. Carbon monoxide has important biological roles across phylogenetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichlorocarbene
Dichlorocarbene is the reactive intermediate with chemical formula CCl2. Although this chemical species has not been isolated, it is a common intermediate in organic chemistry, being generated from chloroform. This bent diamagnetic molecule rapidly inserts into other bonds. Preparation Dichlorocarbene is most commonly generated by reaction of chloroform and a base such as potassium ''tert''-butoxide or aqueous sodium hydroxide. A phase transfer catalyst, for instance benzyltriethylammonium bromide, facilitates the migration of the hydroxide in the organic phase. :HCCl3 + NaOH → CCl2 + NaCl + H2O Other reagents and routes Another precursor to dichlorocarbene is ethyl trichloroacetate. Upon treatment with sodium methoxide it releases CCl2. Phenyl(trichloromethyl)mercury decomposes thermally to release CCl2. :PhHgCCl3 → CCl2 + PhHgCl Dichlorodiazirine, which is stable in the dark, decomposes into dichlorocarbene and nitrogen via photolysis. Dichlorocarbene can also be obta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formamide
Formamide is an amide derived from formic acid. It is a colorless liquid which is miscible with water and has an ammonia-like odor. It is chemical feedstock for the manufacture of sulfa drugs and other pharmaceuticals, herbicides and pesticides, and in the manufacture of hydrocyanic acid. It has been used as a softener for paper and fiber. It is a solvent for many ionic compounds. It has also been used as a solvent for resins and plasticizers. Some astrobiologists suggest that it may be an alternative to water as the main solvent in other forms of life. Formamides are compounds of the type RR′NCHO. One important formamide is dimethylformamide, (CH3)2NCHO. Production Historical production In the past, formamide was produced by treating formic acid with ammonia, which produces ammonium formate, which in turn yields formamide upon heating: :HCOOH + NH3 → HCOO− :HCOO− → HCONH2 + H2O Formamide is also generated by aminolysis of ethyl formate: :HCOOCH2CH3 + NH3 → H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-Butylamine
''tert''-Butylamine is an organic chemical compound with the formula (CH3)3CNH2. It is a colorless liquid with a typical amine-like odor. ''tert''-Butylamine is one of the four isomeric amines of butane, the others being ''n''-butylamine, ''sec''-butylamine and isobutylamine. Preparation ''tert''-Butylamine is produced commercially by direct amination of isobutylene using zeolite catalysts: :NH3 + CH2=C(CH3)2 → H2NC(CH3)3 The Ritter reaction of isobutene with hydrogen cyanide is not useful because it produces too much waste. :(CH3)2C=CH2 + HCN + H2O → (CH3)3CNHCHO :(CH3)3CNHCHO + H2O → (CH3)3CNH2 + HCO2H In the laboratory, it can be prepared by the hydrogenolysis of 2,2-dimethylethylenimine, or via ''tert''-butylphthalimide. Uses ''tert''-Butylamine is used as an intermediate in the preparation of the sulfenamides such as ''N''-''tert''-butyl-2-benzothiazylsulfenamide and ''N''-''tert''-butyl-2-benzothiazylsulfenimide. As rubber accelerators, thes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |