|
Iodine Monofluoride
Iodine monofluoride is an interhalogen compound of iodine and fluorine with formula IF. It is a chocolate-brown solid that decomposes at 0 °C, disproportionating to elemental iodine and iodine pentafluoride: :5 IF → 2 I2 + IF5 However, its molecular properties can still be precisely determined by spectroscopy: the iodine-fluorine distance is 190.9 pm and the I−F bond dissociation energy is around 277 kJ mol−1. At 298 K, its standard enthalpy change of formation is Δ''H''f° = −95.4 kJ mol−1, and its Gibbs free energy is Δ''G''f° = −117.6 kJ mol−1. It can be generated, albeit only fleetingly, by the reaction of the elements at −45 °C in CCl3F: :I2 + F2 → 2 IF It can also be generated by the reaction of iodine with iodine trifluoride at −78 °C in CCl3F: :I2 + IF3 → 3 IF The reaction of iodine with silver(I) fluoride Silver(I) fluoride is the inorganic compound with the formula AgF. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Monochloride
Iodine monochloride is an interhalogen compound with the formula . It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Preparation Iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation : When chlorine gas is passed through iodine crystals, one observes the brown vapor of iodine monochloride. Dark brown iodine monochloride liquid is collected. Excess chlorine converts iodine monochloride into iodine trichloride in a reversible reaction: : Polymorphs has two polymorphs; α-ICl, which exists as black needles (red by transmitted light) with a melting point of 27.2 °C, and β-ICl, which exists as black platelets (red-brown by transmitted light) with a melting point 13.9 °C.Brisbois, R. G.; Wanke, R. A.; Stubbs, K. A.; Stick, R. V. "Iodine Monochloride" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter waves and acoustic waves can also be considered forms of radiative energy, and recently gravitational waves have been associated with a spectral signature in the context of the Laser Interferometer Gravitational-Wave Observatory (LIGO) In simpler terms, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum. Historically, spectroscopy originated as the study of the wavelength dependence of the absorption by gas phase matter of visible light dispersed by a prism. Spectroscopy, primarily in the electromagnetic spectrum, is a fundamental exploratory tool in the fields of astronomy, chemistry, materials science, and physics, allowing the composition, physical structure and e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interhalogen Compounds
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms (fluorine, chlorine, bromine, iodine, or astatine) and no atoms of elements from any other group. Most interhalogen compounds known are binary (composed of only two distinct elements). Their formulae are generally , where ''n'' = 1, 3, 5 or 7, and X is the less electronegative of the two halogens. The value of ''n'' in interhalogens is always odd, because of the odd valence of halogens. They are all prone to hydrolysis, and ionize to give rise to polyhalogen ions. Those formed with astatine have a very short half-life due to astatine being intensely radioactive. No interhalogen compounds containing three or more different halogens are definitely known, although a few books claim that and have been obtained, and theoretical studies seem to indicate that some compounds in the series are barely stable. Some interhalogens, such as , , and , are good halogenating agents. is to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Nitride
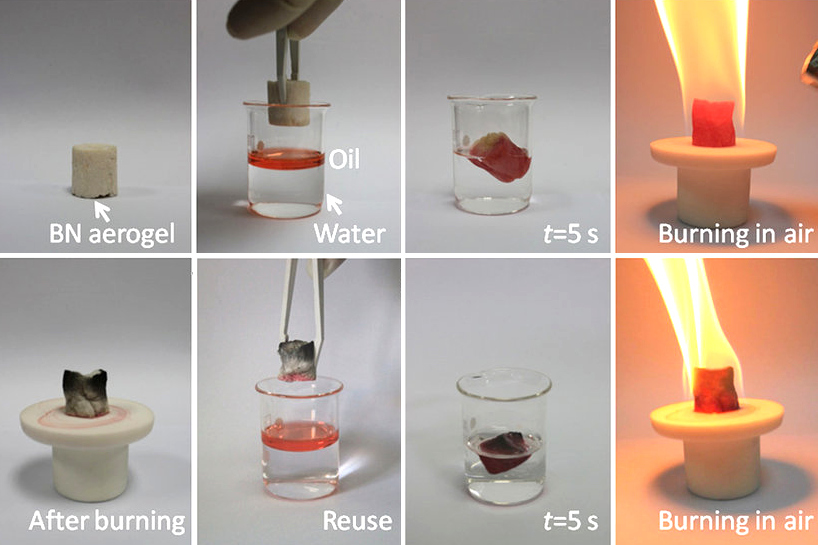
Boron nitride is a thermally and chemically resistant refractory compound of boron and nitrogen with the chemical formula BN. It exists in various crystalline forms that are isoelectronic to a similarly structured carbon lattice. The hexagonal form corresponding to graphite is the most stable and soft among BN polymorphs, and is therefore used as a lubricant and an additive to cosmetic products. The cubic ( zincblende aka sphalerite structure) variety analogous to diamond is called c-BN; it is softer than diamond, but its thermal and chemical stability is superior. The rare wurtzite BN modification is similar to lonsdaleite but slightly softer than the cubic form. Because of excellent thermal and chemical stability, boron nitride ceramics are used in high-temperature equipment and metal casting. Boron nitride has potential use in nanotechnology. Structure Boron nitride exists in multiple forms that differ in the arrangement of the boron and nitrogen atoms, giving rise to varyin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Triiodide
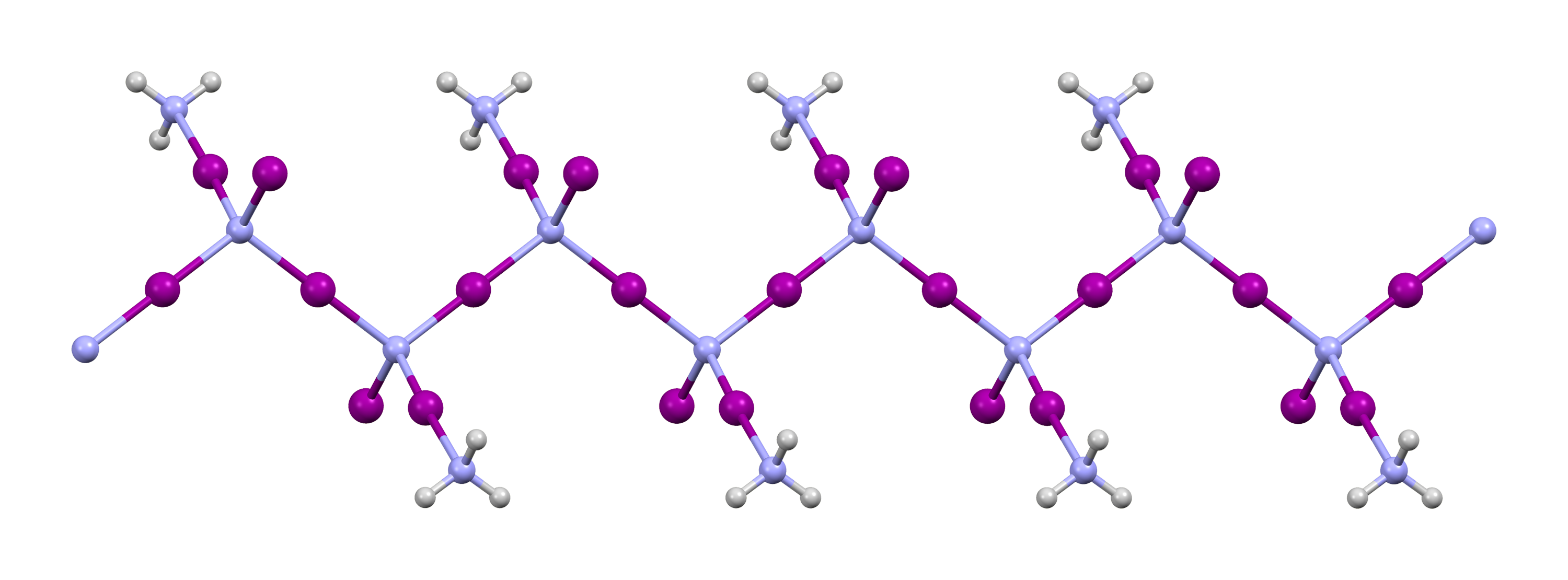
Nitrogen triiodide is an inorganic compound with the formula N I3. It is an extremely sensitive contact explosive: small quantities explode with a loud, sharp snap when touched even lightly, releasing a purple cloud of iodine vapor; it can even be detonated by alpha radiation. NI3 has a complex structural chemistry that is difficult to study because of the instability of the derivatives. Although nitrogen is more electronegative than iodine, the compound was so named due to its analogy to the compound nitrogen trichloride. Structure of NI3 and its derivatives Nitrogen triiodide was first characterized by Raman spectroscopy in 1990 when it was prepared by an ammonia-free route. Boron nitride reacts with iodine monofluoride in trichlorofluoromethane at −30 °C to produce pure NI3 in low yield: :BN + 3 IF → NI3 + BF3 NI3 is pyramidal (C3v molecular symmetry), as are the other nitrogen trihalides and ammonia. The material that is usually called "nitrogen triiodide" is pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver(I) Fluoride
Silver(I) fluoride is the inorganic compound with the formula AgF. It is one of the three main fluorides of silver, the others being silver subfluoride and silver(II) fluoride. AgF has relatively few niche applications; it has been employed as a fluorination and desilylation reagent in organic synthesis and in aqueous solution as a topical caries treatment in dentistry. The hydrates of AgF present as colourless, while pure anhydrous samples are yellow. Preparation High-purity silver(I) fluoride can be produced by the heating of silver carbonate to under a hydrogen fluoride environment, in a platinum tube: :Ag2CO3 + 2 HF -> 2 AgF + H2O + CO2 Laboratory routes to the compound typically avoid the use of gaseous hydrogen fluoride. One method is the thermal decomposition of silver tetrafluoroborate: :AgBF4 -> AgF + BF3 In an alternative route, silver(I) oxide is dissolved in concentrated aqueous hydrofluoric acid, and the silver fluoride is precipitated out of the resulti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Trifluoride
Iodine trifluoride is an interhalogen compound with the chemical formula IF3. It is a yellow solid which decomposes above −28 °C. It can be synthesised from the elements, but care must be taken to avoid the formation of IF5. Reactions F2 reacts with I2 to yield IF3 at −45 °C in CCl3F. Alternatively, at low temperatures, the fluorination reaction I2 + 3 XeF2 → 2IF3 + 3 Xe can be used. Not much is known about iodine trifluoride as it is so unstable. Structure The iodine atom of iodine trifluoride has five electron pairs, of which two are lone-pairs, and the molecule is T-shaped as predicted by VSEPR Theory Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm the .... References {{Fluorine compounds Fluorides Interhalogen compounds Iodine compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichlorofluoromethane
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature. CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer. Historical use Trichlorofluoromethane was first widely used as a refrigerant. Because of its high boiling point (compared to most refrigerants), it can be used in systems with a low operating pressure, making the mechanical design of such systems less demanding than that of higher-pressure refrigerants R-12 or R-22. Trichlorofluoromethane is used as a reference compound for fluorine-19 NMR studies. Trichlorofluoromethane was formerly used in the drinking bird novelty, largely because it has a boiling point of . The replacement, dichloromethane, boiling point , requires a higher ambient temperature to work. Prior to the knowledge of the ozone depletion potential of chlorin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces. The Gibbs energy is the thermodynamic potential that is minim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standard Enthalpy Change Of Formation
In chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of enthalpy during the formation of 1 mole of the substance from its constituent elements in their reference state, with all substances in their standard states. The standard pressure value is recommended by IUPAC, although prior to 1982 the value 1.00 atm (101.325 kPa) was used. There is no standard temperature. Its symbol is . The superscript Plimsoll on this symbol indicates that the process has occurred under standard conditions at the specified temperature (usually 25 °C or 298.15 K). Standard states are as follows: # For a gas: the hypothetical state it would have assuming it obeyed the ideal gas equation at a pressure of 1 bar # For a gaseous or solid solute present in a diluted ideal solution: the hypothetical state of concentration of the solute of exactly one mole per liter (1 M) at a pressure of 1 bar extrapolated from i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and physicist William Thomson, 1st Baron Kelvin (1824–1907). The Kelvin scale is an absolute thermodynamic temperature scale, meaning it uses absolute zero as its null (zero) point. Historically, the Kelvin scale was developed by shifting the starting point of the much-older Celsius scale down from the melting point of water to absolute zero, and its increments still closely approximate the historic definition of a degree Celsius, but since 2019 the scale has been defined by fixing the Boltzmann constant to be exactly . Hence, one kelvin is equal to a change in the thermodynamic temperature that results in a change of thermal energy by . The temperature in degree Celsius is now defined as the temperature in kelvins minus 273.15, meaning t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Dissociation Energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical species. The enthalpy change is temperature-dependent, and the bond-dissociation energy is often defined to be the enthalpy change of the homolysis at 0 K (absolute zero), although the enthalpy change at 298 K (standard conditions) is also a frequently encountered parameter. As a typical example, the bond-dissociation energy for one of the C−H bonds in ethane () is defined as the standard enthalpy change of the process : , : ''DH''°298() = Δ''H°'' = 101.1(4) kcal/mol = 423.0 ± 1.7 kJ/mol = 4.40(2) eV (per bond). To convert a molar BDE to the energy needed to dissociate the bond ''per molecule'', the conversion factor 23.060 kcal/mol (96.485 kJ/mol) for each eV can be used. A variety of experim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |