|
Iodane
Iodane generally refers to any organic derivative of iodine. Without modifier, ''iodane'' is the systematic name for the parent hydride of iodine, HI. Thus, any organoiodine compound with general formula RI (e.g., iodomethane , or iodobenzene ) is a substituted iodane. However, as used in the context of organic synthesis, the term ''iodane'' more specifically refers to organoiodine compounds with nonstandard bond order of bonds between iodine and other atoms, i.e., bond order of iodine greater than 1, making this term a synonym for hypervalent iodine. These iodine compounds are hypervalent because the iodine atom formally contains more than the 8 electrons in the valence shell required for the octet rule. When iodine is ligated to an organic residue and electronegative ligands (e.g. halides or carboxylates), hypervalent iodine occurs in a +3 oxidation state as iodine(III) or λ3-iodane, or in a +5 oxidation state as iodine(V) or λ5-iodane, or in a +7 oxidation state as iodine(VII ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(Diacetoxyiodo)benzene
(Diacetoxyiodo)benzene, also known as phenyliodine(III) diacetate (PIDA) is a hypervalent iodine chemical with the formula . It is used as an oxidizing agent in organic chemistry. Preparation This reagent was originally prepared by Conrad Willgerodt by reacting iodobenzene with a mixture of acetic acid and peracetic acid: :CHI + CHCOH + CHCOH → CHI(OCCH) + HO PIDA can also be prepared from iodosobenzene and glacial acetic acid: :CHIO + 2 CHCOH → CHI(OCCH) + HO More recent preparations direct from iodine, acetic acid, and benzene have been reported, using either sodium perborate or potassium peroxydisulfate as the oxidizing agent: : CH + I + 2 CHCOH + KSO → CHI(OCCH) + KI + HSO + KHSO The PIDA molecule is termed hypervalent as its iodine atom (technically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parent Hydride
In chemistry, a parent hydride in IUPAC nomenclature refers to a main group compound with the formula , where A is a main group element. The names of parent hydrides end with ''-ane'', analogous with the nomenclature for alkanes. Derivatives of parent hydrides are named by appending prefixes or suffixes to the name of the parent hydride to indicate the substituents that replace the hydrogen atoms. Parent hydrides are used in both the organic nomenclature, and inorganic nomenclature systems. (Red Book) Par. IR-6 ''Parent Hydride Names and Substitutive Nomenclature'' - Full text PDF Reactions and structure Parent hydrides are useful reference compounds, but ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodobenzene Dichloride
Iodobenzene dichloride (PhICl2) is a complex of iodobenzene with chlorine. As a reagent for organic chemistry, it is used as an oxidant and chlorinating agent. Chemical structure Single-crystal X-ray crystallography has been used to determine its structure; as can be predicted by VSEPR theory, it adopts a T-shaped geometry about the central iodine atom. Preparation Iodobenzene dichloride is not stable and is not commonly available commercially. It is prepared by passing chlorine gas through a solution of iodobenzene in chloroform, from which it precipitates. The same reaction has been reported at pilot plant scale (20 kg) as well. :Ph-I + Cl2 → PhICl2 An alternate preparation involving the use of chlorine generated ''in situ'' by the action of sodium hypochlorite on hydrochloric acid has also been described. Reactions Iodobenzene dichloride is hydrolyzed by basic solutions to give iodosobenzene (PhIO) and is oxidized by sodium hypochlorite to give iodoxybenzene (PhI ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parent Hydride
In chemistry, a parent hydride in IUPAC nomenclature refers to a main group compound with the formula , where A is a main group element. The names of parent hydrides end with ''-ane'', analogous with the nomenclature for alkanes. Derivatives of parent hydrides are named by appending prefixes or suffixes to the name of the parent hydride to indicate the substituents that replace the hydrogen atoms. Parent hydrides are used in both the organic nomenclature, and inorganic nomenclature systems. (Red Book) Par. IR-6 ''Parent Hydride Names and Substitutive Nomenclature'' - Full text PDF Reactions and structure Parent hydrides are useful reference compounds, but ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peracetic Acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive. Peracetic acid is a weaker acid than the parent acetic acid, with a p''K''a of 8.2. Production Peracetic acid is produced industrially by the autoxidation of acetaldehyde: :O2 + CH3CHO → CH3CO3H It forms upon treatment of acetic acid with hydrogen peroxide with a strong acid catalyst: :H2O2 + CH3CO2H CH3CO3H + H2O As an alternative, acetyl chloride and acetic anhydride can be used to generate a solution of the acid with lower water content. Peracetic acid is generated ''in situ'' by some laundry detergents. This is achieved by the action of bleach activators, such as tetraacetylethylenediamine and sodium nonanoyloxybenzenesulfonate, upon hydrogen peroxide formed from sodium percarbonate in water. The peracetic acid is a more effective bleachi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Square Pyramidal Molecular Geometry
In molecular geometry, square pyramidal geometry describes the shape of certain compounds with the formula where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably . As a transition state in Berry pseudorotation As a trigonal bipyramidal molecule undergoes Berry pseudorotation, it proceeds via an intermediary stage with the square pyramidal geometry. Thus even though the geometry is rarely seen as the ground state, it is accessed by a low energy distortion from a trigonal bipyramid. Pseudorotation also occurs in square pyramidal molecules. Molecules with this geometry, as opposed to trigonal bipyramidal, exhibit heavier ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lone Pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone pairs are found in the outermost electron shell of atoms. They can be identified by using a Lewis structure. Electron pairs are therefore considered lone pairs if two electrons are paired but are not used in chemical bonding. Thus, the number of electrons in lone pairs plus the number of electrons in bonds equals the number of valence electrons around an atom. Lone pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to in the chemistry of Lewis acids and bases. However, not all non-bonding pairs of electrons are considered by chemists to be lone pairs. Examples are the transition metals where the non-bonding pairs do not influence molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apicophilicity
Apicophilicity is the phenomenon in which electronegative substituents of trigonal bipyramidal pentacoordinate compounds prefer to occupy apical (axial) positions (Lap). The term "apicophilicity" was first proposed by Earl L. Muetterties in 1963 for the structural analysis of pentacoordinate phosphorus fluorides by 19F NMR. Since the apical bonding of a pentacoordinate typical (group 1, 2, 13-18) element compound consists of a 3-center-4-electron bond, in which the electron density is localized on two apical substituents, an arrangement in which electronegative substituents occupy apical positions is more stable. The apicophilicity of a substituent is defined as the difference in energy between two isomeric structures in which the substituent occupies an apical position and an equatorial position (Leq). Experimentally, instead of direct measurement of the energy difference, which is usually difficult to measure, the relative energy barriers for pseudorotation In chemistry, a ps ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
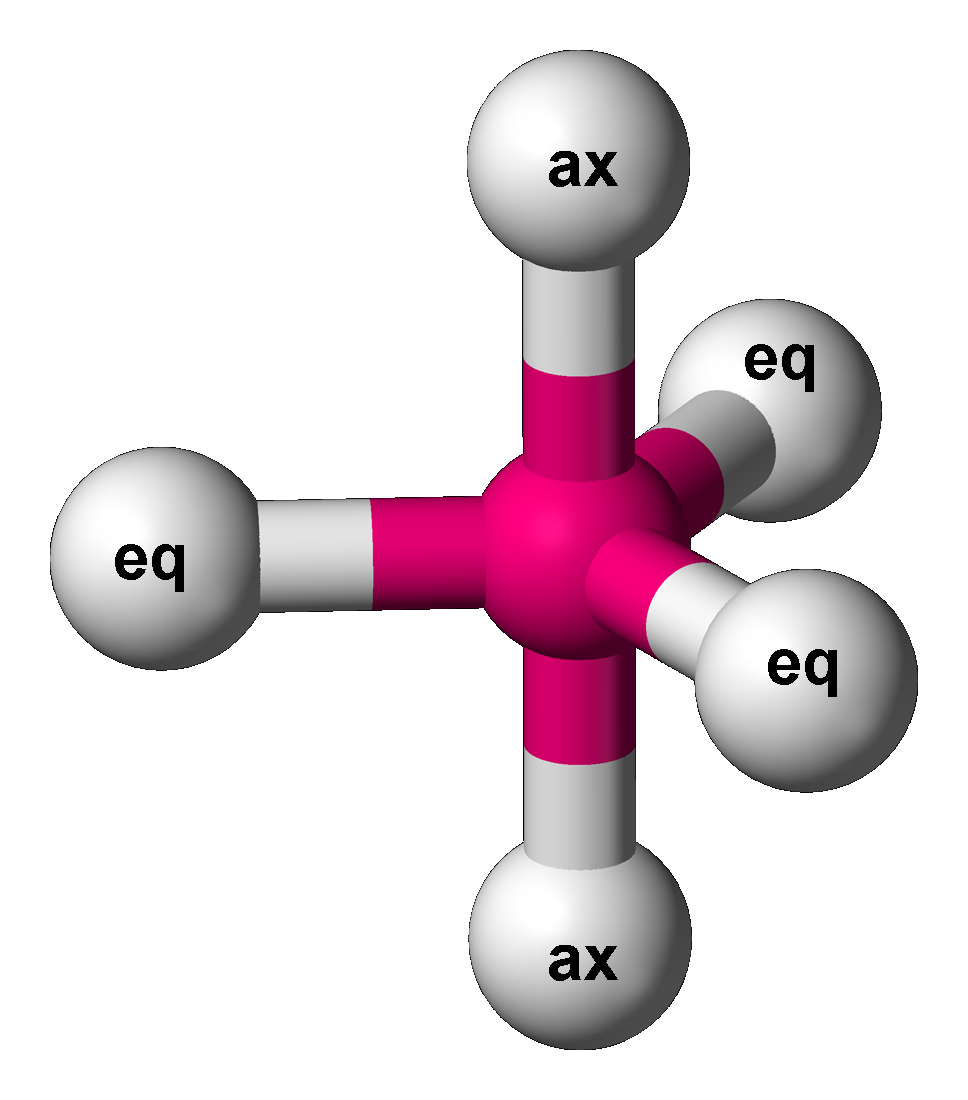
Trigonal Bipyramid Molecular Geometry
In chemistry, a trigonal bipyramid formation is a molecular geometry with one atom at the center and 5 more atoms at the corners of a triangular bipyramid. This is one geometry for which the bond angles surrounding the central atom are not identical (see also pentagonal bipyramid), because there is no geometrical arrangement with five terminal atoms in equivalent positions. Examples of this molecular geometry are phosphorus pentafluoride (), and phosphorus pentachloride () in the gas phase. Axial (or apical) and equatorial positions The five atoms bonded to the central atom are not all equivalent, and two different types of position are defined. For phosphorus pentachloride as an example, the phosphorus atom shares a plane with three chlorine atoms at 120° angles to each other in ''equatorial'' positions, and two more chlorine atoms above and below the plane (''axial'' or ''apical'' positions). According to the VSEPR theory of molecular geometry, an axial position is more crowd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |