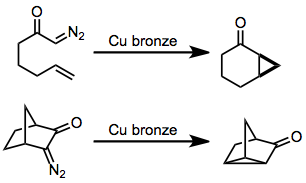
|
Intramolecular Reactions Of Diazocarbonyl Compounds
Intramolecular reactions of diazocarbonyl compounds include addition to alkene, carbon–carbon double bonds to form fused cyclopropanes and insertion into carbon–hydrogen bonds or carbon–carbon bonds. Introduction In the presence of an appropriate transition metal (typically copper or rhodiumDavies, H.; Antoulinakis, E. ''Org. React.'' 2004, ''57'', 1.), diazocarbonyl, α-diazocarbonyl compounds are converted to transition metal carbenes, which undergo addition reactions in the presence of carbon–carbon double bonds to form cyclopropanes. Insertion into carbon–carbon or carbon–hydrogen bonds is possible in substrates lacking a double bond. The intramolecular version of this reaction forms fused carbocycles, although yields of reactions mediated by copper are typically moderate. For enantioselective cyclopropanations and insertions, both copper- and rhodium-based catalysts are employed, although the latter have been more heavily studied in recent years. Mechanism and ste ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds [such as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds, resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and anthraquinone, 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called wikt:quinaquina, quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds and are often re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Salt
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. General properties and reactivity Arenediazonium cations and related species According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group lowers the p''K''a (enhances the acidity) by a million-fold. The stabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protonation
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid, is deprotonation.) Some examples include *The protonation of water by sulfuric acid: *:H2SO4 + H2O H3O+ + *The protonation of isobutene in the formation of a carbocation: *:(CH3)2C=CH2 + HBF4 (CH3)3C+ + *The protonation of ammonia in the formation of ammonium chloride from ammonia and hydrogen chloride: *:NH3( g) + HCl( g) → NH4Cl( s) Protonation is a fundamental chemical reaction and is a step in many stoichiometric and catalytic processes. Some ions and molecules can undergo more than one protonation and are labeled polybasic, which is true of many biological macromolecules. Protonation and deprotonation (removal of a proton) occur in most acid–base reactions; they are the core of most acid–base reaction theories. A Brønst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams, analogous to “R” used for any organic substituent. “Ar” is not to be confused with the elemental symbol for argon. A simple aryl group is phenyl (), a group derived from benzene. Examples of other aryl groups consist of: * The tolyl group () which is derived from toluene (methylbenzene) * The xylyl group (), which is derived from xylene (dimethylbenzene) * The naphthyl group (), which is derived from naphthalene Arylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions. Nomenclature The most basic aryl group is phenyl, which is made up of a benzene ring with one hydrogen atom substituted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(II) Sulfate
Copper(II) sulfate, also known as copper sulphate, is an inorganic compound with the chemical formula . It forms hydrates , where ''n'' can range from 1 to 7. The pentahydrate (''n'' = 5), a bright blue crystal, is the most commonly encountered hydrate of copper(II) sulfate. Older names for the pentahydrate include blue vitriol, bluestone, vitriol of copper,Antoine-François de Fourcroy, tr. by Robert Heron (1796) "Elements of Chemistry, and Natural History: To which is Prefixed the Philosophy of Chemistry". J. Murray and others, Edinburgh. Page 348. and Roman vitriol.Oxford University Press,Roman vitriol, Oxford Living Dictionaries. Accessed on 2016-11-13 It exothermically dissolves in water to give the aquo complex , which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The centers are interconnected by sulfate anions to form chains. Anhydrous copper sulfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intramolecular Cyclization
Intramolecular describes a process or phenomenon that occurs ''within'' a molecule. It can refer to: *Intramolecular reaction Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Examples * intramolecular hydride transfer (transfer of a hy ... * Intramolecular force See also * {{Disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |