|
Interbilayer Forces In Membrane Fusion
Membrane fusion is a key biophysical process that is essential for the functioning of life itself. It is defined as the event where two lipid bilayers approach each other and then merge to form a single continuous structure. In living beings, cells are made of an outer coat made of lipid bilayers; which then cause fusion to take place in events such as fertilization, embryogenesis and even infections by various types of bacteria and viruses. It is therefore an extremely important event to study. From an evolutionary angle, fusion is an extremely controlled phenomenon. Random fusion can result in severe problems to the normal functioning of the human body. Fusion of biological membranes is mediated by proteins. Regardless of the complexity of the system, fusion essentially occurs due to the interplay of various interfacial forces, namely hydration repulsion, hydrophobic attraction and van der Waals forces. Inter-bilayer forces Lipid bilayers are structures of lipid molecules consis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayers
The lipid bilayer (or phospholipid bilayer) is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cell (biology), cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear envelope, nuclear membrane surrounding the cell nucleus, and biological membrane, membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called Ion transporter, ion pump ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potential
Potential generally refers to a currently unrealized ability. The term is used in a wide variety of fields, from physics to the social sciences to indicate things that are in a state where they are able to change in ways ranging from the simple release of energy by objects to the realization of abilities in people. The philosopher Aristotle incorporated this concept into his theory of potentiality and actuality, a pair of closely connected principles which he used to analyze motion, causality, ethics, and physiology in his ''Physics'', ''Metaphysics'', ''Nicomachean Ethics'', and ''De Anima'', which is about the human psyche. That which is potential can theoretically be made actual by taking the right action; for example, a boulder on the edge of a cliff has potential to fall that could be actualized by pushing it over the edge. Several languages have a potential mood, a grammatical construction that indicates that something is potential. These include Finnish, Japanese, and Sanskr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stress (mechanics)
In continuum mechanics, stress is a physical quantity. It is a quantity that describes the magnitude of forces that cause deformation. Stress is defined as ''force per unit area''. When an object is pulled apart by a force it will cause elongation which is also known as deformation, like the stretching of an elastic band, it is called tensile stress. But, when the forces result in the compression of an object, it is called compressive stress. It results when forces like tension or compression act on a body. The greater this force and the smaller the cross-sectional area of the body on which it acts, the greater the stress. Therefore, stress is measured in newton per square meter (N/m2) or pascal (Pa). Stress expresses the internal forces that neighbouring particles of a continuous material exert on each other, while strain is the measure of the deformation of the material. For example, when a solid vertical bar is supporting an overhead weight, each particle in the bar pushe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
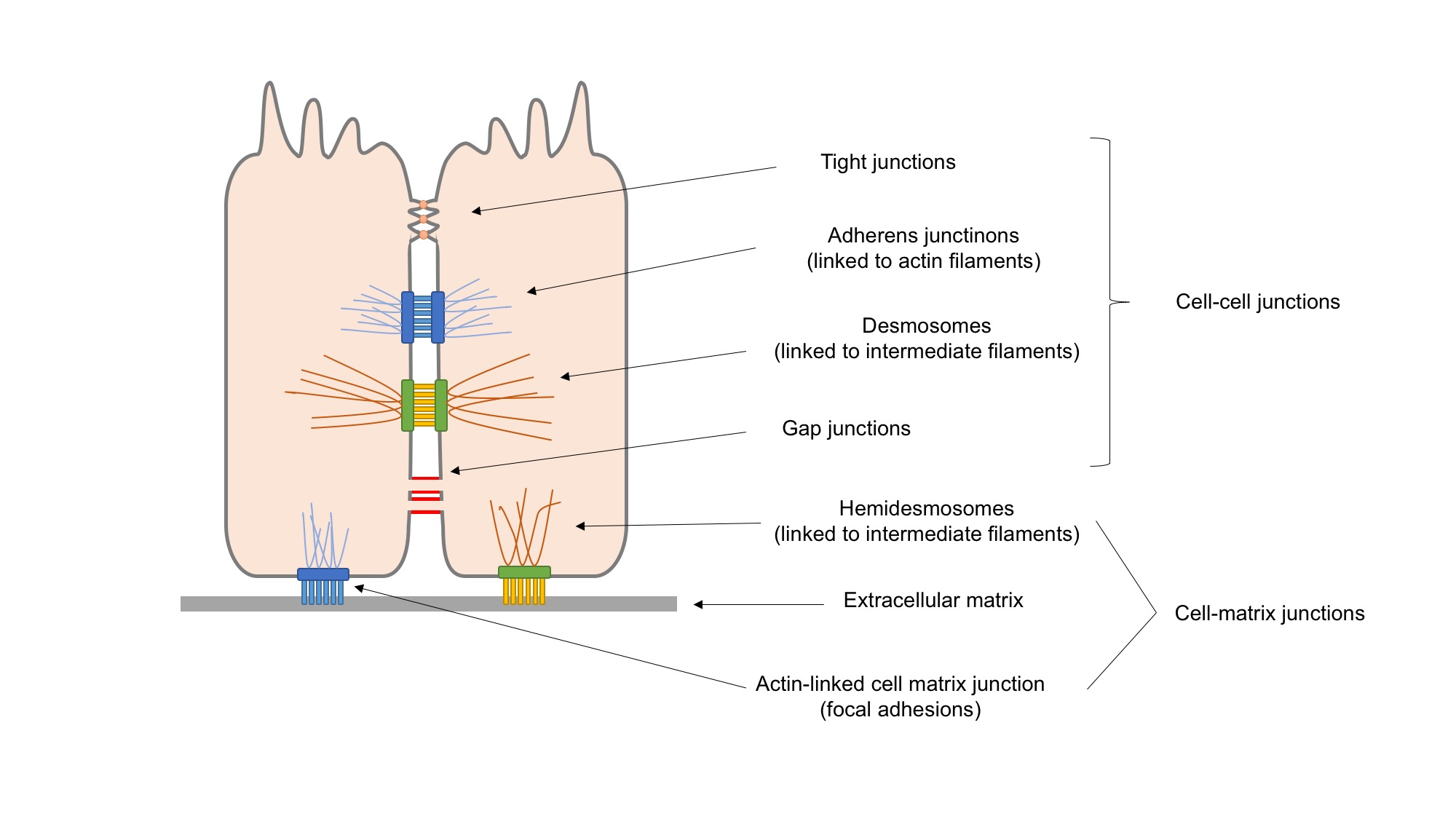
Cell Adhesion
Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesion
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another ( cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are also certain emergent mechanical effects. Surface energy Surface energy is conventionally defined as the work that is required to build an area of a particular surface. Another way to view the surface energy is to relate it to the work required to cleave a bulk sample, creating two surfaces. If the new surfaces are identical, the surface energy γ of each surface is equal to half the work of cleavage, W: γ = (1/2)W11. If the surfac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamaker Constant
The Hamaker constant ''A'' can be defined for a van der Waals (vdW) body–body interaction: :A=\pi^2C\rho_1\rho_2, where \rho_1 and \rho_2 are the number densities of the two interacting kinds of particles, and ''C'' is the London coefficient in the particle–particle pair interaction. It is named after H. C. Hamaker. The magnitude of this constant reflects the strength of the vdW-force between two particles, or between a particle and a substrate. The Hamaker constant provides the means to determine the interaction parameter ''C'' from the vdW-pair potential, w(r) = -C/r^6. Hamaker's method and the associated Hamaker constant ignores the influence of an intervening medium between the two particles of interaction. In 1956 Lifshitz developed a description of the vdW energy but with consideration of the dielectric properties of this intervening medium (often a continuous phase). The Van der Waals forces are effective only up to several hundred angstroms. When the interactions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipole-dipole Interaction
An intermolecular force (IMF) (or secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics. The investigation of intermolecular forces starts from macroscopic observations which indicate the existence and action of forces at a molecular level. These observations include non-ideal-gas thermodynamic behavior reflected by virial coefficients, vapor pressure, viscosity, superficial tension, and absorption data. The first reference to the nature of microscopic for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer Fluid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries, and in nanotechnology. Lipids may be broadly defined as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteins
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coagulation
Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin. Coagulation begins almost instantly after an injury to the endothelium lining a blood vessel. Exposure of blood to the subendothelial space initiates two processes: changes in platelets, and the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to cross-linked fibrin formation. Platelets immediately form a plug at the site of injury; this is called ''primary hemostasis. Secondary hemostasis'' occurs simultaneously: additional coagulation (clotting) factors beyond factor VII ( listed below) respond in a cascade to form fibrin strands, which strengthen the platelet plug. Disorders of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Effect
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_chloride.jpg)